Translate this page into:
Brimonidine-induced punctual eversion: An uncommon adverse effect
*Corresponding author: Riya Prakashbhai Shah, Department of Ophthalmology, M&J Western Regional Institute of Ophthalmology, Ahmedabad, Gujarat, India. shahreeya27@gmail.com
-
Received: ,
Accepted: ,
Read UPDATED-ARTICLE associated with this - 10.25259/GJCSRO_4_1_52
How to cite this article: Shah RP, Chaudhary KG, Bhagat PR. Brimonidine-induced punctual eversion: An uncommon adverse effect. Glob J Cataract Surg Res Ophthalmol. 2024;3:126-8. doi: 10.25259/GJCSRO_27_2024
Abstract
Brimonidine is a highly selective alpha-2 adrenoreceptor agonist which is primarily used to treat glaucoma and ocular hypertension. It has a well-known profile for adverse reactions, the common local ones being periocular dermatitis, allergic conjunctivitis, hyperaemia, and dry eyes. Here, we present two unusual cases of punctal eversion following the administration of brimonidine eye drops. On cessation of the medication, we could haul the progression of the condition. These cases highlight the importance of vigilance on the safety profile of drugs, manifestation of adverse drug reactions, and prompt withdrawal of the offending agent.
Keywords
Brimonidine
Punctual aversion
Glaucoma
Adverse drug reaction
INTRODUCTION
Glaucoma, a leading cause of irreversible blindness worldwide, necessitates a nuanced approach to treatment encompassing various modalities such as anti-glaucoma medications, laser therapy, and surgery. Among these, brimonidine stands as a frequently prescribed agent, functioning as an alpha-2 agonist. Its mechanism of action involves the activation of G protein-coupled receptors, leading to the inhibition of adenylate cyclase activity, thereby reducing cyclic adenosine monophosphate levels and, subsequently, aqueous humour production by the ciliary body. In addition, brimonidine augments uveoscleral outflow by stimulating alpha-adrenergic receptors, thereby promoting prostaglandin release and potentially relaxing the ciliary muscle.[1,2] However, despite its efficacy, brimonidine is not without adverse effects, irrespective of its concentration used. The common ones reported are periocular dermatitis, allergic conjunctivitis, hyperaemia, dry eyes, and blurred vision. Moreover, systemic side effects such as dry mouth and nose, hypotension, headache, and central nervous system depression leading to fatigue, somnolence, sleep apnoea, and mood changes, especially in paediatric patients, have been reported.[3,4] We present here two cases presenting with brimonidine-associated punctal eversion, shedding light on a lesser-known complication in its clinical profile, yet unreported in the literature.
CASE REPORT 1
A 60-year-old male patient, a known case of bilateral moderate primary open-angle glaucoma for 2 years, was regularly instilling travoprost 0.004% eye drops once a day. With a gradual failure to maintain the target intraocular pressure (IOP), brimonidine 0.2% eye drops were added to the regime, to be instilled twice a day. The patient was tolerating the drug well for about 4 months. In a subsequent follow-up at another 3 months, the patient complained of excessive watering and discomfort in both eyes. On examination, mild conjunctival hyperaemia, punctal eversion, and mild medial ectropion were noted [Figure 1]. The adverse reaction was noted, the patient was counselled, brimonidine was discontinued, and another alternative anti-glaucoma medication was initiated. Symptomatic relief was achieved, and punctal eversion resolved in 3 weeks.
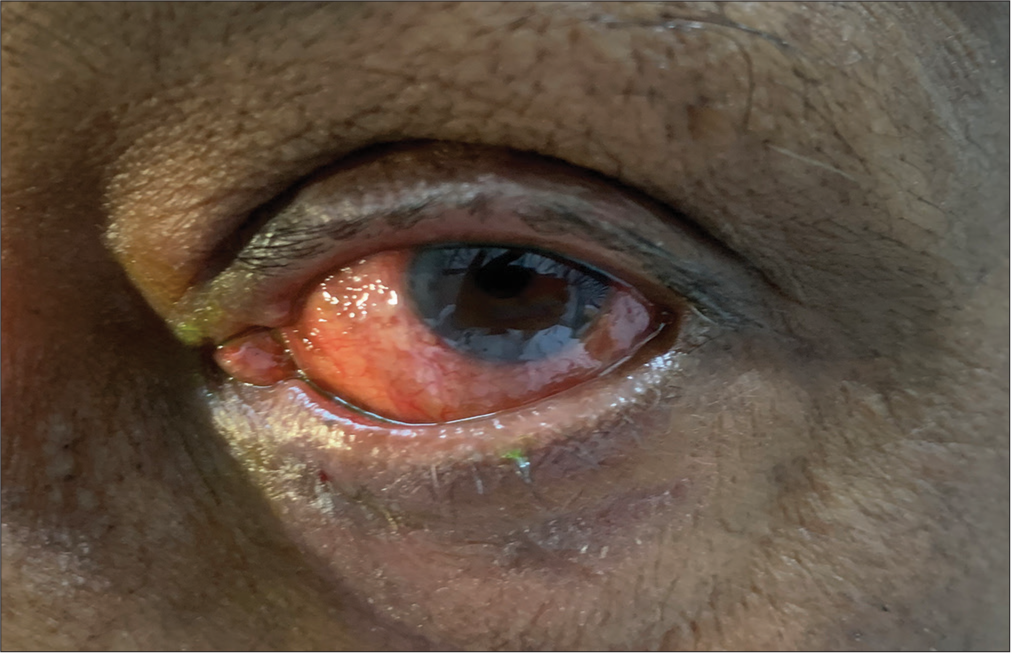
- Lower lid medial ectropion with punctal eversion.
CASE REPORT 2
Another 70-year-old male patient, a known case of bilateral pseudoexfoliative glaucoma, was on latanoprost 0.005% eye drops once a day and brimonidine 0.15% eye drops twice a day for 1 year. In one of the follow-ups, this patient also complained of watering, itching, dryness, and irritation in both eyes. On examination, periocular dermatitis with punctal eversion was noted in both eyes [Figures 2 and 3].
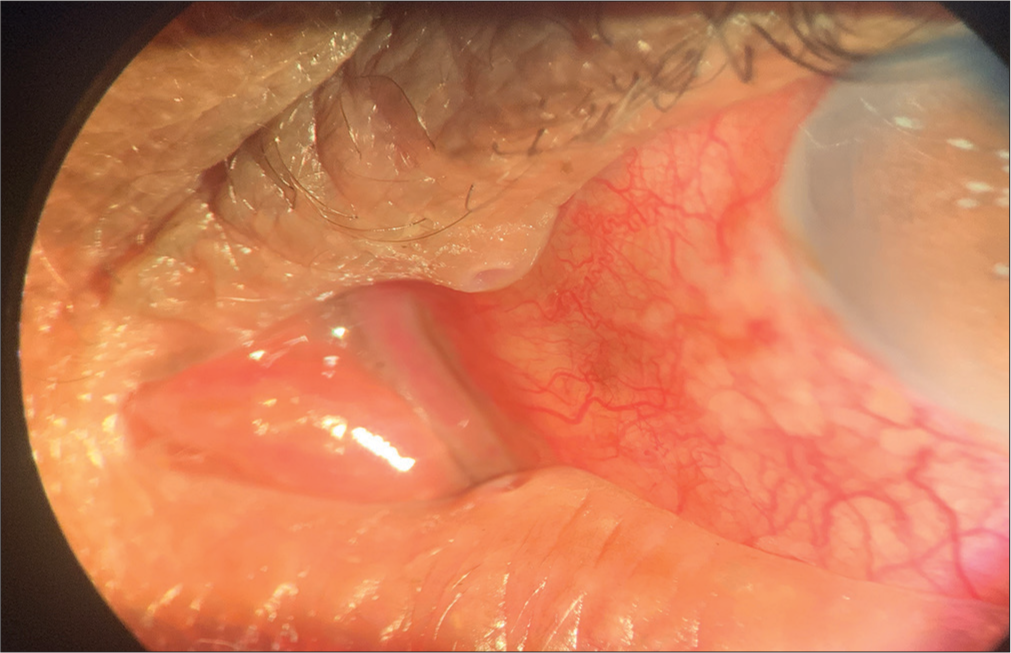
- Punctal eversion.
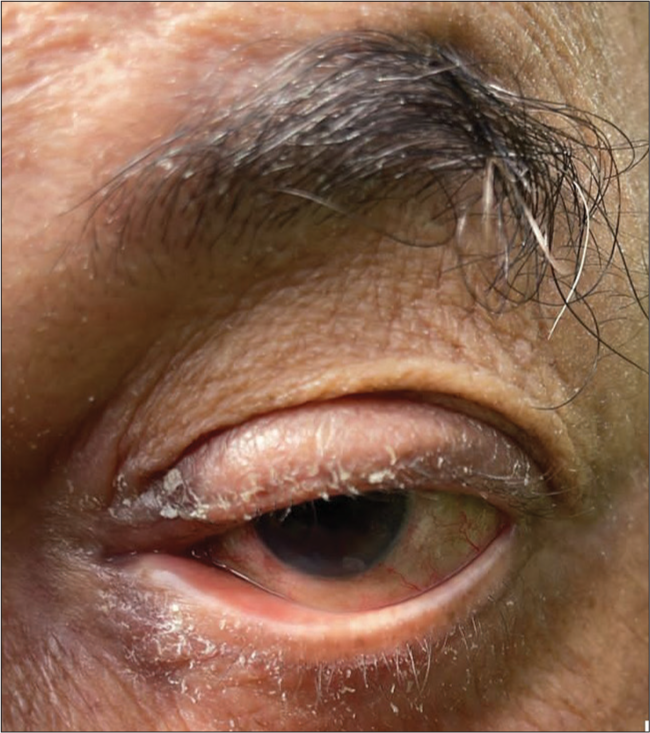
- Periocular dermatitis with lower lid ectropion and punctal eversion.
Brimonidine was discontinued, and the patient was shifted to another equivalent anti-glaucoma medication. The adverse reactions abated in 3 weeks.
DISCUSSION
Medical treatment forms a core component of the glaucoma management spectrum. The success of treatment depends, to a significant extent, on its tolerability and acceptability. Tolerability and compliance are, in turn, dependent on the comfort perceived by the patient while using the drugs.[5] In general, topical anti-glaucoma drugs have a reasonably acceptable safety profile. The incidence of side effects increases with increasing age of the patient, increased duration of therapy and increasing number of medications.[5] Although few, these side effects can affect the quality of life of the patient and compliance, which can be limiting factors for the success of treatment. Brimonidine is a widely prescribed IOP-lowering agent. Despite its efficacy, brimonidine is associated with several side effects that warrant attention and consideration in clinical practice.[3,4] The ocular surface is particularly vulnerable to the adverse effects of brimonidine. Patients may experience symptoms such as ocular irritation, dryness, and allergic conjunctivitis. These effects can be attributed to the vasoconstrictive properties of the drug, which may compromise tear film stability and exacerbate preexisting dry eye conditions. In addition, allergic reactions to brimonidine or its preservatives can manifest as conjunctival hyperaemia and itching, further impacting patient comfort and adherence to therapy. Systemic absorption of the drug can also lead to central nervous system and cardiovascular adverse effects such as dizziness, fatigue, somnolence, headache, hypotension and bradycardia. Periorbital dermatitis, characterised by erythema, oedema, pruritus, and excoriation around the eyes, has been reported as a noteworthy side effect of brimonidine. While the exact pathogenesis is not fully understood, it is hypothesised to result from a hypersensitivity reaction to the medication or its preservatives. The periorbital skin is particularly sensitive and susceptible to irritants, making it prone to inflammatory reactions triggered by topical medications like brimonidine. Mechanical ectropion and eventually punctal eversion is an uncommonly known complication observed following chronic use of brimonidine topical formulation. Chronic exposure to brimonidine leads to cicatricial changes in the anterior lamella of the lid in susceptible individuals and can manifest as contact dermatitis and tissue oedema, leading to cicatricial ectropion and punctal eversion.[6,7] Dermatitis-induced itching can also invoke frequent rubbing near the lids, skin excoriation, and a gradual ectropion. The breach in the integrity of the lacrimal apparatus results in excessive lacrimation and irritation. Clinicians should maintain a high index of suspicion, especially in patients presenting with new-onset periocular symptoms following initiation of brimonidine therapy. Early recognition of this condition and discontinuation of therapy is of paramount importance as it may facilitate complete resolution.[6] Appropriate treatment for dermatitis may be initiated in severe cases in consultation with a dermatologist.
Topical corticosteroids and emollients may be needed to alleviate the inflammation and restore skin barrier function. Patients on brimonidine who develop cicatricial ectropion should not be managed surgically at their first presentation.[7]
CONCLUSION
While brimonidine is an effective medication for glaucoma, its use is associated with a range of potential side effects. Brimonidine-associated ectropion and punctal eversion is an uncommon adverse event that can occur at any point of time during the therapy. Early recognition and management to mitigate discomfort and complications are warranted. Prescribers should be vigilant in monitoring patients for these adverse events, understanding their mechanisms, and implementing appropriate strategies to ensure optimal therapeutic outcomes while minimising risks.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr Purvi Raj Bhagat is on the editorial board of the Journal.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Effects of brimonidine on aqueous humor dynamics in human eyes. Arch Ophthalmol. 1995;113:15147.
- [CrossRef] [PubMed] [Google Scholar]
- Brimonidine: A new alpha2-adrenoreceptor agonist for glaucoma treatment. J Glaucoma. 1997;6:250-8.
- [CrossRef] [PubMed] [Google Scholar]
- Periocular allergic contact dermatitis caused by brinzolamide. Contact Dermatitis. 2021;84:274-6.
- [CrossRef] [Google Scholar]
- Ocular and systemic side effects of Brimonidine 0.2% eye drops (Alphagan) in children. Eye (Lond). 2004;18:24-6.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation and comparison of the safety profile of topical anti-glaucoma drugs in patients of primary open angle glaucoma or ocular hypertension or normal tension glaucoma. Clin Exp Vis Eye Res J. 2020;3:17-29.
- [CrossRef] [Google Scholar]
- Drug-induced ectropion: What is best practice? Ophthalmology. 2007;114:362-6.
- [CrossRef] [PubMed] [Google Scholar]
- Reversible cicatricial ectropion precipitated by topical brimonidine eye drops. Ophthalmic Plast Reconstr Surg. 2008;24:57-8.
- [CrossRef] [PubMed] [Google Scholar]







