Translate this page into:
Should test for dry eyes be done before age-related cataract surgery?
*Corresponding author: Modupe Balogun, Department of Ophthalmology, Lagos State University Teaching Hospital, Lagos, Nigeria. dupebal@yahoo.co.uk
-
Received: ,
Accepted: ,
How to cite this article: Balogun M, Saka I. Should test for dry eyes be done before age-related cataract surgery? Glob J Cataract Surg Res Ophthalmol. doi: 10.25259/GJCSRO_41_2024
Abstract
Objectives:
This study aimed to determine if tests for dry eyes should be done routinely before age-related cataract surgery.
Materials and Methods:
This was a hospital-based observational prospective study. Patients 50 years and above with age-related cataracts that met the inclusion criteria scheduled for manual small incision cataract surgery (MSICS) were assessed for dry eye disease (DED) before and after surgery. Numerical variables were described using the mean and standard deviation (SD) with a 95% confidence interval and P < 0.05.
Results:
A total of 81 patients were studied: 60.5% (49) males and 39.5% (32) females. The mean pre-operative Schirmer’s test value was 14.09 mm (SD = 10.7 mm). Post-operative schirmer’s test values were 18.12mm (SD = 11.81mm) at 1 week, 14.98mm (SD = 11.26mm) at 4 weeks and 16.20 mm (SD = 11.06 mm) at 6 weeks. Pre-operative mean tear break-up time (TBUT) was 10.05 (SD = 5.13 s), 9.46 s (SD = 4.18 s) at 1 week, 10.13 s (SD = 4.29 s) at 4 weeks and 9.80 s (SD = 4.33 s) at 6 weeks postoperatively. Lissamine green staining score (LGSS) Grade 0 was 80% at 1 week post-operative, Grade 0 increased to 85%, 90% at 4 weeks and 91% at post-operative 6 weeks.
Conclusion:
This study showed that DED is not associated with MSICS, and it is not associated with objective worsening of previously diagnosed DED. Testing for DED preoperatively plays a significant role in the pre-operative evaluation of patients and symptomatic expectations, but its testing before MSICS might not always be necessary.
Keywords
Dry eye disease
Cataract surgery
Ocular surface
INTRODUCTION
Cataract
A cataract is an opacity of the crystalline lens of the eye, causing various levels of visual impairment and blindness as a result of changes to the transparency and refractive index of the lens. It is a leading cause of blindness globally, responsible for 51% of blindness.[1-4]
The prevalence of cataracts increases with age, ranging from 3.9% among 55–64 years to 92.6% among those 80 years and older.[5] The prevalence of blindness due to cataracts ranges from 5% in the United States of America and the United Kingdom to 55% in some parts of Africa and 58.5% in Peru and Australia.[6] The Nigerian National Blindness Survey reported that cataract was the most common cause of severe visual impairment and blindness and were responsible for 45.3% and 43%, respectively.[7] Given the ageing population and increase in life expectancy, the number of people affected with cataracts is predicted to increase worldwide, especially in low-income nations with limited access to surgery.[5]
The mainstay for the treatment of cataracts is surgery, as there are no preventive or therapeutic drugs yet approved to clear the cataract.[5] Cataract surgery has been shown to be one of the most cost-effective healthcare interventions[8], and uneventful cataract surgery with the implantation of an intraocular lens is highly effective in visual improvement.[9] Uneventful cataract surgery improves the functional and psychological outcome of the patient, particularly after both eye surgeries.[10,11]
Cataract surgeries have since evolved from couching, which dates back to the fifth century BC,[12] to Intracapsular Cataract Extraction and Extracapsular Cataract Extraction (ECCE). The modern-day version of ECCE, known as manual small incision cataract surgery (MSICS), is now used in many parts of the world, especially in developing countries. Phacoemulsification cataract surgery is the preferred method in the developed world with the smaller incision resulting in a more stable anterior chamber throughout surgery and shorter recovery time.[12] In developing countries, the most efficient and economical means of managing cataracts is MSICS with corneoscleral tunnel incision.[13] The procedure is fast with a low rate of complications, and can be performed on all types of nuclei.[13]
Dry eye disease (DED)
The Tear Film and Ocular Surface Society International Dry Eye Workshop (TFOS DEW II) in 2017 defined dry eye as a multifactorial disease of the ocular surface characterised by a loss of homeostasis of the tear film, accompanied by ocular symptoms in which tear film instability and hyperosmolarity, ocular surface inflammation and damage and neurosensory abnormalities play etiological roles.[14,15] The global prevalence of DED was estimated at 29.5% using the TFOS DEW II diagnostic criteria[16], with women more affected at 28.1% than men at 24.9%.[16] In the United States of America, the prevalence of DED in the adult population was 6.8% and increased with age.[17] The overall prevalence of DED in Africa was 42.0%, and in Nigeria, it was 41.4%[18], while in South-West Nigeria, it was estimated that 32.5% of adults above 40 years have DED.[19] It is estimated that dry eye occurs more frequently among elderly and postmenopausal women[20]; this may be a result of lacrimal gland function, which reduces as we age, and non-environmental factors such as medications as antihistamines, hormone replacement therapy, and meibomian gland degeneration, leading to reduce or inadequate oil production and meibomian gland dysfunction.
The burden of DED can have an emotional and psychological impact on the individual and also a financial burden on society. DED can affect the vision and quality of life of the individual as symptoms interfere with daily activities such as reading, writing, or working on video display monitors.[21] The patient can become frustrated with the course of treatment, repeated hospital visits, and specialist visits seeking treatment changes or may seek alternative treatments leading to significant utilisation of medical resources and funds.[20]
DED and cataract surgery
The impact of dry eyes on cataract surgery includes delayed healing and vision recovery, large optical aberration, and inaccurate intraocular lens calculation, thus increasing the risk of postoperative adverse outcomes, complications and infections.[22,23] Cataract surgery may induce or worsen DED, thus reducing patient satisfaction after successful surgery.[24,25]
Various studies published on dry eye in cataract patients undergoing phacoemulsification and MSICS showed that DED could be exacerbated by these surgeries[13,23,26-28], and symptoms and signs of dry eyes occurred as early as 7 postoperative days while severity pattern improved over time.[27] The corneal incision made at the time of surgery disrupts the feedback mechanism that stimulates the lacrimal gland by the corneal nerves.[29] Prolonged exposure to the microscope light, aggressive intraoperative irrigation of the tear film, and frequent topical anaesthetic on the day of surgery all contribute to trauma to either the cornea epithelium or conjunctiva mucous goblet cell loss or both.[29] The use of a povidone-iodine solution to sterilise the surgical field can elevate inflammatory markers from ocular surface damage, contributing to the mechanism of DED after cataract surgery.[23]
MATERIALS AND METHODS
This study was conducted at the eye institute of the Lagos State University Teaching Hospital. It was a hospital-based observational prospective study involving 81 participants from June to October 2023. The study populations were adult patients 50 years and above with age-related cataracts that met the eligibility criteria and were examined by an ophthalmologist to be scheduled for MSICS with an intraocular lens implant. All participants preoperatively were questioned about dry eye-related symptoms such as pricking, burning, and itching sensations, and the observations were noted, following which slit-lamp examination was done, Schirmer’s test-1, lissamine green staining and tear film break-up time were evaluated. All participants gave their consent voluntarily to participate in this study.
The inclusion criteria for this study were patients 50 years and above diagnosed with age-related cataracts and scheduled for MSICS. Exclusion criteria were patients <50 years of age and cataracts caused by aetiologies other than age (such as trauma, uveitis, drugs, and metabolic disorders like diabetes). Patients with pre-existing ocular diseases of the conjunctiva, cornea, lids and chemical burns, patients on chronic ocular medications, allergies, pterygium, blepharitis or medications that can cause dry eyes such as antihistamines, antidepressants, and decongestants, were excluded from the study.
Participants completed an interviewer-administered questionnaire designed for this research. Participants had an anterior segment examination followed by Schirmer 2 test (with anaesthesia) using the Whatman 41 test strip. The wet area between 0 and 5 mm was considered as severe, 5–10 mm as moderate, while 10–15 mm as mild dry eyes. Greater than 15 mm of wetting was considered a normal tear function. Tear break-up time (TBUT) was assessed using fluorescein strips. Three TBUT readings were taken and the average was calculated. TBUT of <10 s was indicative of the presence of dry eyes. Lissamine green test was done preoperatively and postoperatively and graded using the modified Oxford scale. The ocular surface disease index (OSDI) and dry eye severity grading scheme (DEWS 2007) questionnaire was completed by each participant before surgery.
Schirmer’s test, TBUT and lissamine green test were done on all the recruited patients on 1 day before surgery, 1st week, 4 weeks and 6 weeks post-surgery.
Ethical approval to conduct the study was obtained from the Health Research Ethics Committee of Lagos State University Teaching Hospital.
RESULTS
A total of 81 patients were studied. While 60.5% (49) were male, 39.5% (32) were female. In terms of age classification, 19.8% were 50–59 years, 49.4% were 60–69 years, 27.2% were 70–79 years and 2.5% were 80–89 years, while 1.2% were 90– 99 years [Table 1].
| Gender | Age | |||||
|---|---|---|---|---|---|---|
| Male | Female | 50–59 years | 60–69 years | 70–79 years | 80–89 years | 90–99 years |
| Frequency | ||||||
| 49 | 32 | 16 | 40 | 22 | 2 | 1 |
| Percentage | ||||||
| 60.5 | 39.5 | 19.8 | 49.4 | 27.2 | 2.5 | 1.2 |
In this study, Schirmer’s test value of ≥15 mm was taken as normal, 15–10 mm as mild, 10–5 mm as moderate grade and 5–0 mm as severe dry eye. The mean Schirmer’s test value was 14.09 mm with standard deviation (SD) = 10.7 mm, with the lowest value being 0.5 mm and the highest value being 35 mm preoperatively. After 1 week postoperatively, the mean Schirmer’s test value increased to 18.12 mm with SD = 11.81 mm. In the 4th week, the mean Schirmer’s test value came down to 14.98 mm with SD = 11.26 mm, and it increased to 16.20 mm with SD = 11.06 mm in the 6th week [Table 2 and Figure 1].
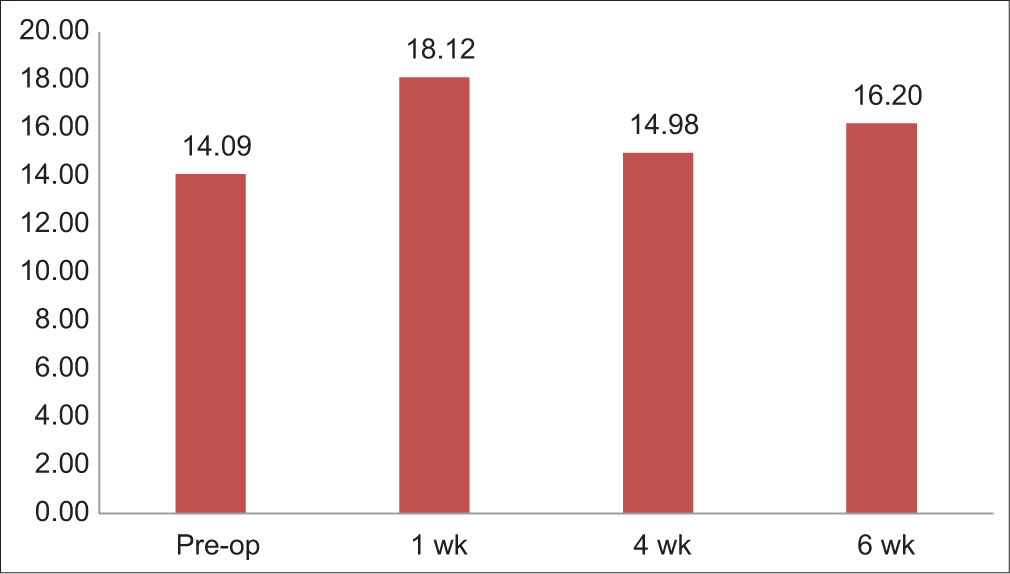
- Schirmer’s test 1-value changes.
| Tests | Pre-op | 1 week | 4 week | 6 week |
|---|---|---|---|---|
| Schirmer | 14.09±10.71 | 18.12±11.81 | 14.98±11.26 | 16.20±11.06 |
| TBUT | 10.05±5.13 | 9.46±4.18 | 10.13±4.29 | 9.80±4.33 |
Preoperatively, Schirmer’s test analysis showed 44.4% of patients had normal values, 3.7% had mild grades, 22.2% had moderate and 29.6% had severe grades. However, after 1 week, 58.7% patients had normal value, 8.0% had mild grade, 13.3% had moderate and 20.0% had severe grade. The differences in Schirmer’s test values between pre-operative and post-operative 1 week were clinically significant with P = 0.015 [Table 3].
| Normal (%) | Mild (%) | Moderate (%) | Severe (%) | P-value | |
|---|---|---|---|---|---|
| Pre-operative | 44.4 | 3.7 | 22.2 | 29.6 | Pre-op versus others |
| 1st week | 58.7 | 8.0 | 13.3 | 20.0 | 0.015 |
| 4th week | 43.8 | 10.0 | 16.3 | 30.0 | 0.459 |
| 6th week | 51.3 | 10.3 | 14.1 | 24.4 | 0.122 |
After a 4-week post-operative period, Schirmer’s test values showed 43.8% normal, 10.0% mild grade, 16.3% moderate and 30.0% severe grade. The difference between pre-operative values and post-operative 1-month values was not clinically significant with P = 0.459 [Table 3]. After the 6-week’ post-operative period, Schirmer’s test values showed 51.3% normal, 10.3% mild grade, 14.1% moderate and 24.4% severe grade. The difference between pre-operative values and post-operative 6-week values was clinically not significant with P = 0.122 [Table 3].
In this study, tear break-up time of more than 10 s was taken as normal, between 9 and 7 s as mild, between 7 and 5 s as moderate and <5 s as severe. Preoperatively, the mean tear break-up time TBUT was 10.05 s with SD = 5.13 s with a minimum value of 2 s and a maximum value of 29 s. The time decreased to 9.46 s with SD = 4.18 s with a minimum value of 3 s and a maximum of 21 s at 1 week post-operative. On 4 weeks’ post-operative, it was 10.13 s with SD = 4.29 s with a minimum value of 4 s and a maximum of 21 s. After post-operative 6 weeks, it became 9.80 s with SD = 4.33 s with a minimum value of 4 s and a maximum of 24 s [Table 2 and Figure 2].
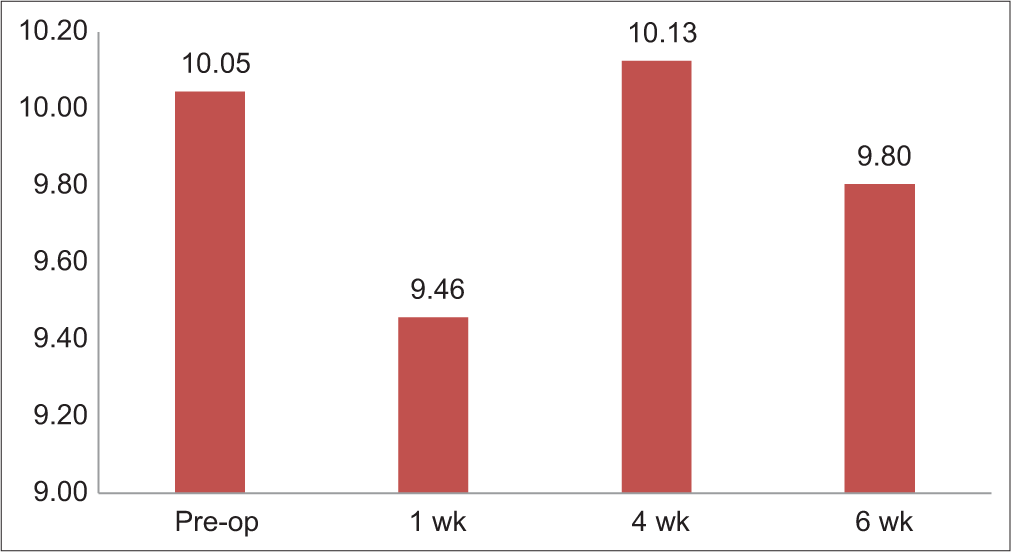
- Tear break-up time (TBUT)-changes overtime.
Preoperatively, 35.8% had normal values, 34.6% had mild dry eyes, 23.5% had moderate dry eyes, and 6.2% had severe dry eyes. Post-operative 1 week, 38.7% had normal value, 30.7% had mild dry eyes, 20.0% had moderate dry eyes and 10.7% had severe dry eyes. After 4 weeks, 38.8% had normal eyes, 41.3% had mild, 13.8% had moderate and 6.3% had severe dry eyes. After 6 weeks postoperatively, 33.8% had normal eyes, 41.3% had mild, 16.3% had moderate and 8.8% had severe dry eyes. However, the difference between TBUT pre-operative and post-operative 1 week, 4 weeks and 6 weeks were clinically insignificant [Table 4]. Preoperatively, lissamine green staining score (LGSS) was more of Grade 0, with Grade 0 found to be 80%, Grade 1: 7%, Grade 2: 11% and Grade 3: 1%. After 1 week post-operative, Grade 0 increased to 85%, then increased to 90% in 4 weeks’ post-operative, and it was 91% after post-operative 6 weeks [Table 5].
| Normal (%) | Mild (%) | Moderate (%) | Severe (%) | P-value | |
|---|---|---|---|---|---|
| Pre-operative | 35.8 | 34.6 | 23.5 | 6.2 | Pre-op versus others |
| 1st week | 38.7 | 30.7 | 20.0 | 10.7 | 0.260 |
| 4th week | 38.8 | 41.3 | 13.8 | 6.3 | 0.885 |
| 6th week | 33.8 | 41.3 | 16.3 | 8.8 | 0.697 |
| Grades | Pre-operative | 1 week | 4 week | 6 week |
|---|---|---|---|---|
| Grade 0 | 80 | 85 | 90 | 91 |
| Grade 1 | 7 | 7 | 3 | 1 |
| Grade 2 | 11 | 7 | 6 | 8 |
| Grade 3 | 1 | 1 | 1 | 0 |
After applying the comprehensive grading system of dry eyes, 96.3% of patients had dry eyes postoperatively. Among the patients, 8.6% have a mild grade, 23.5% have moderate and 64.2% have a severe grade [Table 6 and Figure 3]. Analysis by gender showed 47 (60.3%) male patients and 31 (39.7%) female patients had dryness of the eye.
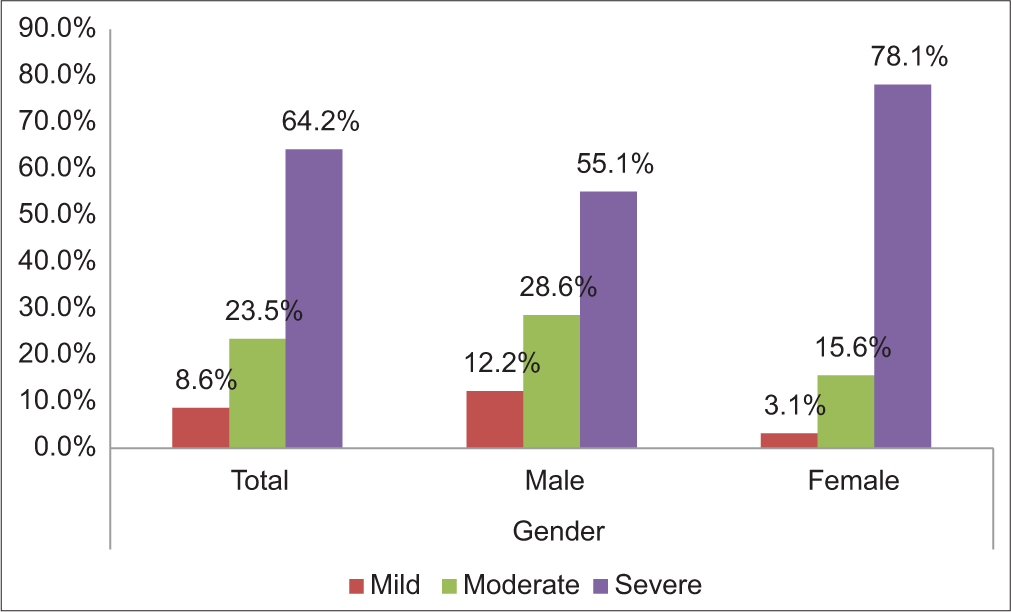
- Severity of dry eyes according to ocular surface disease index.
| Total | Male | Female | ||||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | Frequency | % | |
| Normal | 3 | 3.7 | 2 | 4.1 | 1 | 3.1 |
| Mild | 7 | 8.6 | 6 | 12.2 | 1 | 3.1 |
| Moderate | 19 | 23.5 | 14 | 28.6 | 5 | 15.6 |
| Severe | 52 | 64.2 | 27 | 55.1 | 25 | 78.1 |
OSDI: Ocular surface disease index
DISCUSSION
DED is a common finding following small incision cataract surgery.[13,26,28] The diagnostic relevance of Schirmer’s test strip and the TBUT in the classification of normal and moderate DED was high as they both showed a positive correlation in these two categories. From the total number of study participants, the number of participants with mild DED increased from the 1st week to the sixth post-operative week, as assessed by the objective Schirmer’s test strip. A similar increase in the number of participants classified under mild DED using TBUT was noted, mostly in the 4th and 6th weeks. However, mild and severe DED cases showed significant diagnostic disparity with Schirmers and TBUT from this study. Contrary findings were observed from Li et al.[24] study where DED assessment using Schirmers and TBUT showed a positive correlation at different phases of assessment.[24] Sample size might explain this disparity as Li et al. had 37 patients assessed in their study and severity grading was not reported in each phase. Another source of disparity may be due to the fact that Schirmer’s test is a grossly objective measure, whereas TBUT is susceptible to observer subjectivity in determining the earliest patch of dry spot on the cornea being examined.[24]
The increase in Schirmer’s test values at 1 week post-operative period is that this study is thought to be due to the frequent instillation of topical post-operative medications, which increase tear volume and tear lake, thus improving Schirmer’s test values. Patients in the study had topical steroids and antibiotic drops applied at a frequency of either hourly or 2 hourly. Symptomatic mild dry eye can be secondary to the increase in particulate matter in either topical eye drops or topical preservatives inducing ocular surface disturbance or both, increasing the number of participants with conjunctiva staining with lissamine green and mild dry eye in this study. Naderi et al. [28], in their study, concluded that DED can be exacerbated by cataract surgery. Patil et al.[30] reviewed two major etiological risk factors for DED following MSICS: preservative in topical medications and damage to corneal nerves during surgery.
At the 6-week post-operative period, the incidence of severe DED had reduced significantly among participants, and over half (51.3%) of the respondents had normal Schirmer’s test values, compared to 44.4% preoperatively. Saif et al.,[26] in their study, demonstrated similar findings but only at 3 months, where there was no statistically significant difference with baseline pre-surgery values.[26] Jayshree et al.,[31] in their study, also found that TBUT and Schirmer’s test values at 30 days were not clinically significant compared to pre-operative values.[31] When a comparison of TBUT and lissamine green staining scores was done, comparable findings with the Schirmer’s were found as no statistically significant changes in values were observed preoperatively and at 6 weeks post-operative period.
Subjective assessment of DED using questionnaires from this study differed significantly from the objective dry eye assessment methods. Severe dry eye was reported in 64.2% of respondents, compared to 29.6% using the objective Schirmer’s test strip and 6.2% with the tear film break-up time test. Similar findings were observed, where the OSDI questionnaire showed a weak but statistically significant negative correlation with the non-invasive tear film breakup time.[32,33] This proves that DED is largely a symptomatic disease, and objective methods of assessing dryness are essential in making a clear diagnosis of the disease.
CONCLUSION
This study shows that DED following MSICS is not associated with the objective worsening of DED. Testing for DED preoperatively plays a significant role in the pre-operative evaluation of patients and symptomatic expectation, but testing for dry eye before MSICS might not always be necessary.
Ethical approval
Ethical approval to conduct the study was obtained from the Health Research Ethics Committee of Lagos State University Teaching Hospital (LASUTH), number LREC/06/10/2226, dated August 17, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Cataracts in adults: Management London: National Institute for Health and Care Excellence (NICE); 2017.
- [Google Scholar]
- Factors affecting cataract surgical coverage and outcomes: A retrospective cross-sectional study of eye health systems in sub-Saharan Africa. BMC Ophthalmol. 2015;15:67.
- [CrossRef] [Google Scholar]
- Cataract surgical coverage and visual outcome using RAAB in Birnin Gwari local government Area, North West Nigeria. Niger J Basic Clin Sci. 2020;17:91-6.
- [CrossRef] [Google Scholar]
- Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614-8.
- [CrossRef] [Google Scholar]
- Global, regional, national burden and gender disparity of cataract: Findings from the global burden of disease study 2019. BMC Public Health. 2022;22:2068.
- [CrossRef] [Google Scholar]
- Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844-51.
- [Google Scholar]
- Causes of blindness and visual impairment in Nigeria: The Nigeria national blindness and visual impairment survey. Invest Ophthalmol Vis Sci. 2009;50:4114-20.
- [CrossRef] [Google Scholar]
- Impact of cataract surgery on quality of life in Plateau State, Nigeria. Niger J Ophthalmol. 2009;17:5-10.
- [CrossRef] [Google Scholar]
- The impact of cataract surgery on vision-related quality of life for bilateral cataract patients in Ho Chi Minh City, Vietnam: A prospective study. Health Qual Life Outcomes. 2014;12:16.
- [CrossRef] [Google Scholar]
- Evaluation of dry eye before and after manual small incision cataract surgery in Bundelkhand region: Comparative study. IOSR J Dent Med Sci. 2019;18:48-54.
- [Google Scholar]
- Dry eye after cataract surgery and associated intraoperative risk factors. Korean J Ophthalmol. 2009;23:65-73.
- [CrossRef] [Google Scholar]
- Definition and diagnostic criteria of dry eye disease: Historical overview and future directions. Invest Ophthalmol Vis Sci. 2018;59:DES7-12.
- [CrossRef] [Google Scholar]
- The global prevalence of dry eye disease: A Bayesian view. Ophthalmic Physiol Opt. 2021;41:1254-66.
- [CrossRef] [Google Scholar]
- Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90-8.
- [CrossRef] [Google Scholar]
- Prevalence of dry eye disease in Africa: A systematic review and meta-analysis. Optom Vis Sci. 2020;97:1089-98.
- [CrossRef] [Google Scholar]
- Dry eye disease in an adult population in South-West Nigeria. Cont Lens Anterior Eye. 2016;39:359-64.
- [CrossRef] [Google Scholar]
- The relative burden of dry eye in patients' lives: Comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci. 2005;46:46-50.
- [CrossRef] [Google Scholar]
- Dry eye disease: Prevalence, assessment, and management. Home Healthc Now. 2018;36:74-83.
- [CrossRef] [Google Scholar]
- Dry eye management in patients after cataract surgery: A literature review. Int J Nurs Health Serv. 2019;2:340-5.
- [Google Scholar]
- Preoperative optimization of ocular surface disease before cataract surgery. J Cataract Refract Surg. 2017;43:1596-607.
- [CrossRef] [Google Scholar]
- Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea. 2007;26(9 Suppl 1):S16-20.
- [CrossRef] [Google Scholar]
- The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:75-92.
- [CrossRef] [Google Scholar]
- Dry eye changes after phacoemulsification and manual small incision cataract surgery (MSICS) Int J Ophthalmol Eye Res. 2016;4:184-91.
- [CrossRef] [Google Scholar]
- Cataract surgery and dry eye disease: A review. Eur J Ophthalmol. 2020;30:840-85.
- [CrossRef] [Google Scholar]
- The unsatisfied patient after cataract surgery ocular surface disease as a major contributor! Int J Ophthalmol Clin Res. 2018;5:95.
- [CrossRef] [Google Scholar]
- An insight into the role of various factors involved in the pathogenesis of dry eyes after manual small-incision cataract surgery: A mini review. Ann SBV. 2019;8:51-3.
- [CrossRef] [Google Scholar]
- A prospective study of dry eye after manual small incision cataract surgery in rural population of Bagalkot. J Clin Res Ophthalmol. 2017;4:25-9.
- [Google Scholar]
- Noninvasive tear breakup times and ocular surface disease. Optom Vis Sci. 2013;90:1086-91.
- [CrossRef] [Google Scholar]
- Comparison of ocular-surface disease index questionnaire, tear film break-up time, and Schirmer tests for the evaluation of the tear film in computer users with and without dry-eye symptomatology. Clin Ophthalmol. 2012;6:1303-6.
- [CrossRef] [Google Scholar]






