Translate this page into:
Minimally invasive glaucoma surgery: A comprehensive review
*Corresponding author: Suneeta Dubey, Department of Glaucoma, Dr. Shroff ’s Charity Eye Hospital, New Delhi, India. dubeysuneeta@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dubey S, Drishti C. Minimally invasive glaucoma surgery: A comprehensive review. Glob J Cataract Surg Res Ophthalmol. 2024;3:92-101. doi: 10.25259/GJCSRO_34_2024
Abstract
Minimally Invasive Glaucoma Surgery (MIGS) has emerged as a major advancement in the management of glaucoma, providing a less invasive and safer alternative to traditional surgeries. Conventional glaucoma surgeries are typically reserved for patients with advanced glaucoma who are on “maximally tolerated medical therapy” or those with advanced disease. However, there is often a tendency to delay surgery because of the risk of potential complications of these invasive surgeries. MIGS is characterized by a high safety profile, with a lower risk of severe complications compared to traditional glaucoma surgeries. It is particularly suitable for patients with mild-to-moderate open-angle glaucoma. MIGS tends to bridge the therapeutic gap between medical management and traditional invasive surgeries. MIGS encompasses a variety of techniques and devices, such as Trabectome, iStent, Kahook Dual Blade, Gonioscopy-Assisted Transluminal Trabeculotomy (GATT), Hydrus, and minimally invasive bleb surgeries like PreserFlo Microshunt and Xen Implant. Post-operative recovery is generally quicker, with many patients experiencing improvements in their quality of life due to reduced medication dependence and faster healing.However, like all other interventions, MIGS is not without potential complications. Transient hyphema, IOP spikes, and device-related issues can occur. This review classifies MIGS procedures based on their anatomical targets, which include trabecular meshwork bypass, suprachoroidal space, and subconjunctival filtration.It explores the mechanisms, approaches, and clinical outcomes associated with different types of MIGS. A comprehensive literature search using PubMed was conducted, studies published between January 2000 and March 2023 were thoroughly read to gather insights into the evolving terminology, indications, contraindications, and classification of MIGS procedures. In conclusion, MIGS offers a promising alternative for glaucoma management, especially for patients with mild-to-moderate disease. Its safety, rapid recovery, and ability to reduce medication burdens make it a valuable addition to glaucoma armamentarium. However, further research and long-term studies are needed to fully establish the efficacy and safety of these innovative techniques.
Keywords
Glaucoma
Intraocular pressure
Schlemm’s canal
Trabecular meshwork
Minimally invasive glaucoma surgery devices
INTRODUCTION
Glaucoma is a chronic progressive optic neuropathy that is characterised by optic nerve head cupping and changes in the visual field. The most important modifiable risk factor for the development and progression of glaucoma is intraocular pressure (IOP).
In mild-to-moderate glaucoma, the conventional treatment modalities include pharmacotherapy and lasers such as iridotomy and laser trabeculoplasty. The treatment for advanced stage is usually surgical procedures such as trabeculotomy and glaucoma drainage devices (GDDs).[1]
Unlike cataract surgeries, glaucoma surgeries are not 1-time procedures. They require rigorous post-operative follow-up to manage various early and late complications, and repeated interventions due to the gradual decline in the procedure’s efficacy over time. Despite advancements in surgical techniques and the development of new procedures, the outcomes of glaucoma surgery remain suboptimal. This ongoing challenge has led to the introduction of numerous innovations and devices in the market as researchers and clinicians strive for the ideal glaucoma surgery.
In the past two decades, minimally invasive glaucoma surgeries (MIGSs) have emerged as a viable surgical option to address many unmet needs in the treatment of glaucoma.[2] MIGS involves a wide array of surgical techniques and devices that aim to lower IOP with a higher level of safety than traditional glaucoma surgeries.[3,4] A key characteristic of these procedures is that they utilize an ab interno approach with rapid recovery time and preserve the conjunctiva as a protection against future glaucoma outbreaks.
METHODOLOGY
A thorough literature search was performed utilising the PubMed database to identify pertinent studies on MIGS published in English. The search strategy employed terms and keywords including iStent, trabecular stent, sclerotomy, Schlem’s canal (SC) canalisation, gonioscopy-assisted transluminal trabeculotomy, Trabectome, CyPass, Kahook Dual Blade (KDB), Hydrus, SC dilatation, XEN, Ab Interno Canaloplasty (AbiC), suprachoroidal shunt, Tanito microhook, suprachoroidal stent, InnFocus and EX-PRESS. To ensure full coverage and incorporation of all pertinent publications, combinations of both free text keywords and Medical Subject Headings terms were utilised.
In addition to the primary search, reverse snowballing of the reference lists was done to identify any additional relevant study carefully. The search was conducted from 1 January 2000 to 1 March 2023 to gather the most recent data in the fast-growing field of MIGS. Whereas, conference abstracts were excluded from the study.
TERMINOLOGY
As MIGS continues to evolve, so understanding the terms associated with these procedures and devices is essential for clear, universal communication among ophthalmologists, patients and researchers.[5]
Ab interno procedure involves an internal approach through a clear corneal incision, while ‘ab externo’ procedures are performed through an external approach. These procedures typically require an incision in the sclera or conjunctiva.
MIGS devices can be classified into subconjunctival filtration, supraciliary shunts and trabecular meshwork (TM) bypass based on the site of action. Bypassing the TM in the conventional outflow pathway, TM bypass devices such as Hydrus and iStent aim to enhance aqueous humour outflow. A suprachoroidal shunt such as the iStent Supra and CyPass Micro-Stent exits the eye through the supraciliary space, providing an alternate pathway for the aqueous humour. Subconjunctival filtration devices such as XEN Gel Stents and PreserFlo MicroShunts facilitate drainage to the subconjunctival space from the anterior chamber (AC) and allow absorption of aqueous humour in the conjunctiva and episcleral vessels.[6]
MIGS CHARACTERISTICS
High safety profile : MIGS offers high safety with minimal intraoperative and postoperative complications.
Minimum disruption of angle anatomy: MIGS procedures should improve the physiologic aqueous drainage without altering normal angle anatomy
Ab interno procedure: Through ab interno approach under direct visualisation of the angle
Moderately efficacious: IOP reduction is usually less than filtering surgeries but should still be at least 20%
MIGS offers ease to both patients and surgeons – minimal bedtime with excellent post-operative recovery.[6]
INDICATIONS
MIGS procedures should be considered in the following situations:
Early glaucoma: These procedures are usually indicated in the management of patients with mild-moderate glaucoma. The patients who failed to achieve target pressures through Pharmacotherapy.[2,7,8]
Primary open-angle glaucoma (POAG).
Pigmentary glaucoma.
Pseudoexfoliation glaucoma (PXFG).
Noncompliance to treatment adverse drug reaction with the medical management.[4]
MIGS can be performed in conjunction with cataract surgery. Thus reducing the number of surgeries and anti-glaucoma medication load thereby improving patient satisfaction and overall quality of life (QOL).[4,9]
CONTRAINDICATIONS
MIGS contraindications depend on the patient’s clinical characteristics and the device/procedure being employed.[9]
However, a few common contraindications for MIGS are as follows:
Advanced glaucoma, that is glaucoma with advanced field loss (Hodapp-Parrish-Anderson (HPA) Classification).
Glaucoma in uveitis
Neovascular glaucoma (NVG): Active rubeotic glaucoma
Primary/secondary angle-closure glaucoma. However, MIGS procedures can be performed if the angles open post peripheral iridotomy/goniosynechialysis.
Corneal pathology.
Elevated episcleral vasculature pressure, for example, thyroid eye disease, Sturge Weber syndrome and retrobulbar tumour.[10]
MIGS acting on the TM, like iStent, might be contraindicated in patients with peripheral anterior synechiae (PAS) due to the potential risk of progressive synechial closure postoperatively.
CLASSIFICATION
Basic classification of MIGS has been depicted in Figure 1. Based on their surgical site, MIGSs are classified as:

- Classification of minimally invasive glaucoma surgery.
TM Bypass
The primary site of resistance to aqueous outflow is juxtacanalicular TM. MIGS techniques address this by ablating or excising the TM or bypassing it with stents, facilitating direct aqueous humour drainage into Schlemm’s canal.[11]
This approach is suitable for patients POAG, PXFG, ocular hypertension and pigmentary glaucoma, aiming for a target pressure of mid-teens. However, such procedures are contraindicated in cases of primary angle-closure glaucoma, NVG, corneal opacity, angle dysgenesis and high episcleral venous pressure glaucoma.[12]
Complications of TM bypass can include IOP spike hyphema, obstruction, malposition and, displacement of the stent and PAS formation.[13]
STENTS
iStent (Glaukos, Laguna Hills, CA)
The iStent, the first Food and Drug Administration-approved MIGS device, was introduced to the market in 2012. As Shown in Figure 2 this tiny stent is made of heparin-coated titanium, measuring 1 mm in length and 0.3 mm in width, with a 0.25 mm high snorkel having a central lumen of 120 mm that projects into the AC. Designed with a ridge shaped like a snorkel and featuring three arches for retention, the iStent ensures adequate placement in the nasal angle of SC.[14]

- Istent Glaukos (image courtesy: The cleveland clinic).
An ab interno Implantation of iStent is performed through a clear corneal incision with a pre-loaded inserter under direct gonioscopy. Spiegel et al. observed a reduction of approximately 25% IOP in about 70% of patients, thereby significantly decreasing medication burden. Placement of multiple iStents can lead to an IOP reduction of 40–44%, while a single stent can achieve a 20–27% reduction in IOP within 1 year.[15]
iStent inject (Glaukos, Laguna Hills, CA)
Approved by the Food and drug administration (FDA) in 2018, this second-generation TM bypass stent, like its predecessor the iStent, is a small medical implant made of heparin-coated, non-ferromagnetic titanium. As shown in Figure 3, the stent measures 360 mm in height and 230 micrometres in diameter, with a lumen of 80 mm. The stent has a tapered end called the head, which sits in SC, while the thorax extends into the trabecular meshwork and AC.[16]

- Istent inject (image courtesy: Dr. John Behrdal).
The iStent inject has two stents and comes with a preloaded injector system (G2-M-IS injector). It is implanted using an ab interno approach under direct gonioscopy. It is placed nasally into the TM and Schlemm’s canal, spaced 30–60° apart.[17]
According to an analysis by Lavia et al., the iStent inject results in a 35–39% reduction in IOP and decreases the need for medications, with 65–75% of patients being medication-free after 1 year.[6] In addition, studies have shown that the reduction in IOP achieved with the implantation of two stents is comparable to the reduction achieved with two medications after 1 year.[18]
iStent inject® W
iStent Inject® W is a third-generation device developed by Glaukos to achieve a better positioning of iStent. Figure 4a shows the basic structure of istent Inject w and Figure 4b shows intraoperative image of istent Inject W implantation. This device has the same height as the second-generation iStent with a broader base diameter of 360 µm for better drainage.[14]

- (a) iStent inject® W (Courtesy: Glaukos) and (b) intraoperative gonioscopic image of iStent inject® W (Courtesy: Dr SD, SCEH).
Hydrus stent (Ivantis Inc, Irvine, CA, USA)
Hydrus implant, as shown in Figure 5 is a small, crescent-shaped stent measuring 290 µm in diameter and 8 mm in length. It is made of nitinol, known best for its shape memory; it features an inlet with three openings that rest in the AC. With a preloaded injector, it is usually implanted in the inferonasal/inferotemporal quadrant through a clear corneal incision.[19]

- Hydrus Microstent (image courtesy: Vince Giuseffi).
The stent has been designed with intracanalicular scaffolds and occupies nearly 120-150 degrees of SC sparing the posterior portion of the collector channel. It operates through a unique trimodal mechanism: Bypassing the TM to allow aqueous flow, dilating SC by 4–5 times to facilitate outflow through collector channels and enhancing overall aqueous humour drainage.[20]
The HORIZON study found that 80% of cases achieved more than 20% IOP reduction. About 73% of eyes were drug-free at 24 months.[20] The COMPARE study, which compared the Hydrus stent to the iStent, concluded that 39.7% of eyes Hydrus experienced more than a 20% IOP decrease, compared to 13.3% in iStent. Additionally, approximately 46.6% of patients with the Hydrus stent were AGM-free, compared to 24% with iStent.[21]
TISSUE EXCISION (TRABECULOTOMY)
Kahook dual blade(KDB)
It was launched in 2015. It can be performed stand-alone or with phacoemulsification. It utilises a single-use disposable blade with a sharp tip. A clear corneal incision is made, and the KDB is inserted. Its pointed tip pierces TM and enters SC. The ramp lifts and stretches TM, and during circumferential movement in the canal, parallel blades excise a strip of the TM, exposing the outer wall of Schlemm’s canal. This strip of TM can be removed using aspiration or intraocular forceps.
This technique facilitates minimal TM tissue damage.
Figure 6a shows the basic structure and Figure 6b shows the intraoperative image of KDB. Dorairaj and Tam reported significant reductions in IOP and the number of medications needed in patients with angle-closure glaucoma.[22]

- (a) Kahook dual blade (KDB) (Courtesy: AAO) and (b) intraoperative gonioscopy of KDB (Courtesy: DR.SD, SCEH).
Trabectome
Introduced by Baervelt, Chuck and Irvine, Trabectome got FDA certified in 2004 For the management of paediatric and adult glaucoma. It utilises a plasma-mediated ablation system with continuous irrigation and aspiration and a bipolar 550 Hz electrode. It creates an opening between the anterior chamber, Schlemm’s canal and the collector channels. Figure 7a shows the basic structure and Figure 7b shows the intraoperative image of Trabectome. According to a study by Dubey et al., the overall success rate with the trabectome was 76% in Indian patients.[23]
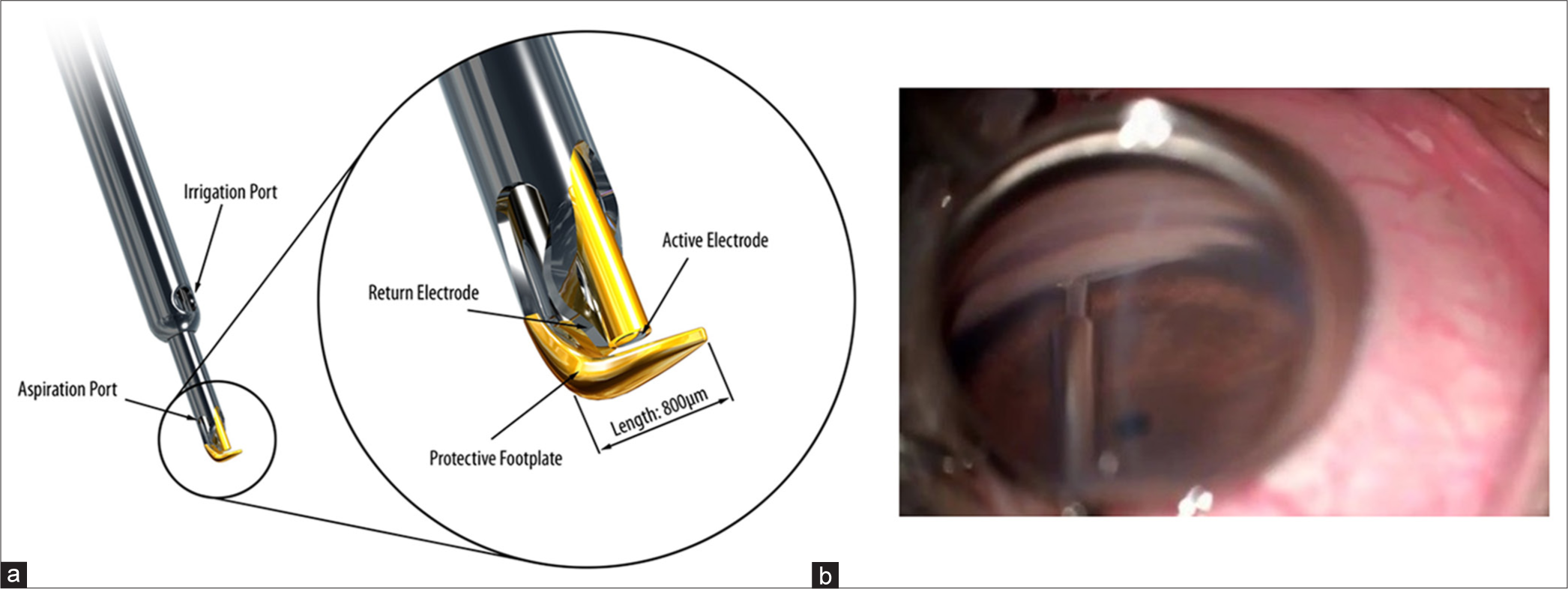
- (a) Trabectome (Courtesy: Prof Guss Guzzard, Moorfields Eye Hospital). (b) Intra operative gonioscopy image of Trabectome (Courtesy: Dr SD, SCEH).
Laser Trabeculostomy
First described by Berlin in 1987 and utilised by Vogel and Lauritzen in 1997, this technique employs an excimer laser with a fibre optic delivery system. It uses an 80-nanosecond pulse of 308 nm xenon chloride laser to target TM. Each energy pulse delivers 1.2 mJ for 10–60 nanoseconds at a 20 Hz frequency. This procedure effectively removes the tissue obstructing the aqueous drainage with minimal damage to the surrounding.
10 microchannels spaced 500 µm apart are created over a 90-° area. This process is also referred to as pneumatic cranioplasty. The procedure typically results in a reduction of IOP between 20 and 40%.[24]
Gonioscopy-assisted transluminal trabeculotomy (GATT)
In 2014, Grover et al.[25] described this technique which involves microsurgical forceps and an iTrack microcatheter. It has a shaft diameter of 200 µm, featuring a distal tip with an illuminating catheter for location monitoring. Under direct gonioscopy, the suture or microcatheter is guided toward the nasal quadrant into SC and is then advanced 360° with the help of microforceps. Traction is applied to break the TM leading to 360° trabeculotomy.[26]
GATT has demonstrated effectiveness in approximately 70–90% of cases, with an IOP reduction ranging from 30 to 40%, compared to the ab externo approach.[27] Figure 8 shows intraoperative image of 360 degrees Suture GATT.
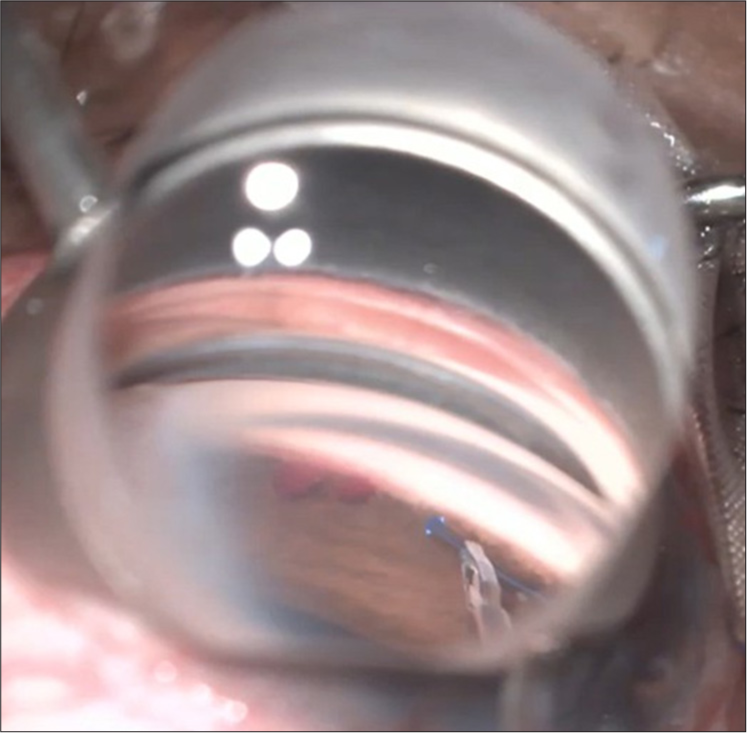
- Suture gonioscopy assisted transluminal trabeculotomy (Courtesy: Dr. SD, SCEH).
Tanito ab interno micro-hook trabeculotomy
The tip of a modified sinskey-like instrument called Tanito microhook is introduced into the SC and swept circumferentially over the required clock hours (varies from 180 to 270°) to incise the inner wall of SC and TM. Figure 9 depicts the intraoperative image of tanito microhook trabeculotomy.

- Ab interno trabeculotomy with Tanito Microhook (Courtesy: intraoperative gonio image on Tanito Microhook, Courtesy : Dr Masaki Tanito, MD).
Maheshwari et al. study showed a complete surgical success of 89.1% at the end of 1 year in the phaco-trabeculotomy group.[28]
AbiC
Interno Canaloplasty (AbiC) is facilitated by the iTrack microcatheter, which boasts a 200-µm thickness, featuring a bulbous 250-µm tip and a lubricating coating for smooth passage. The device is equipped with a visco-injector and fibre optic enabled illumination which aids in navigating through the SC. Following catheter insertion and positioning, it is withdrawn, allowing for the insertion of a Visco surgical device. The Visco surgical device is instrumental in viscodilating areas of resistance, including the Schlemm canal and collector channels. Through viscodilation, perforations within the TM are created, thereby enhancing aqueous outflow. This technique typically results in a 30–40% reduction in IOP over 12 months, often necessitating fewer than two anti-glaucoma medications for effective management.[29]
SUPRACHOROIDAL SHUNTS
These devices aid in directing aqueous humour flow to the suprachoroidal space in a controlled way, thereby increasing uveoscleral pathway outflow. This establishes a continuous path between AC and supraciliary space.
Cypass stent
Figure 10 shows the cypass stent. FDA approved in 2016, the Cypass Stent is a flexible, fenestrated polyamide stent measuring 6.35 mm × 510 µm, with a lumen of 300 µm. It is a pre-loaded device with a guidewire to assist in shaping the sclera for dissection and insertion between the AC and suprachoroidal space.[30] However, in 2018, it was withdrawn from the market due to concerns regarding increased loss of endothelial cells.[31]

- Cypass (Courtesy: San Jose Eye institute).
iStent supra
Made of biocompatible polyethersulfone, this curved device has a heparin-coated tube with a lumen of 0.16 mm. It features a titanium sleeve and retention ridge for stabilisation. Mounted with a guide for easy insertion into both the AC and suprachoroidal space via an ab interno approach under gonioscopic guidance. When used in conjunction with Travoprost, iStent Supra has been demonstrated to reduce IOP by 20% in advanced glaucoma patients.[32] Figure 11 depicts the structure of iStent supra.

- iS’tent Supra (Courtesy: Glaukos).
SUBCONJUNCTIVAL FILTRATION SURGERIES (MINIMALLY INVASIVE BLEB SURGERY)
Xen implant (Xen Gel Stent)
This FDA-approved pre-loaded device comes with a disposable 27G injector composed of porcine collagen-derived gelatine cross-linked with glutaraldehyde and is hydrophilic in nature.[33] In the dry state, it is rigid and straight and becomes flexible when hydrated. It measures 6 mm in length with an internal lumen diameter of 45 µm and an external diameter of 150 µm. Available in three variations: XEN 140, XEN 63 and XEN 45. Typically implanted in the superonasal or superior quadrant.[34]
InnFocus MicroShunt (PRESERFLO MicroShunt)
It comprises two segments: a longer proximal part measuring 4.5 mm and a shorter distal part measuring 3 mm. It is Constructed from poly (styrene-block- isobutylene-block-styrene) or SIBS. Features an external lumen diameter of 350 µm and an internal lumen diameter of 75 µm. According to Batlle et al., patients receiving the shunt with mitomycin C (MMC) exhibited controlled IOP within the low teens for up to 3 years post-implantation. The Preserflo MicroShunt is implanted through an ab externo approach and performed under topical anaesthesia. The procedure involves creating a small conjunctival peritomy, followed by the preparation of a sub-tenon pocket treated with MMC. Subsequently, irrigation is performed, and a scleral tunnel is created, into which the device is inserted parallel to the iris plane. The Tenon layer and conjunctiva are then closed to facilitate bleb formation without leaks.[35]
POST-OPERATIVE COURSE AND QOL
As compared to traditional glaucoma surgery, MIGS procedures usually have a predictable and favourable postoperative course, with minimal discomfort and fast recovery. Due to their minimally invasive nature and preservation of conjunctival and scleral tissue, these procedures are less prone to complications.
In most cases, patients return to a normal routine within a week after MIGS, although strenuous activities should be avoided in the early post-operative period. Following surgery, patients typically receive topical anti-inflammatory medications and antibiotics and are advised regular follow-up visits, IOP monitoring and recovery assessment.[36]
MIGS primarily aims to reduce IOP, which is crucial for preventing or halting glaucoma progression. Although MIGS has shown decent efficacy in IOP Lowering, the efficacy is usually lesser compared to traditional glaucoma surgeries such as trabeculotomy and GDDs. However, the IOP achieved is often adequate for managing early glaucoma.
A key advantage of MIGS is the safety profile. The risk of vision-threatening complications such as hypotony, infection or suprachoroidal haemorrhage is relatively rare compared to traditional glaucoma surgeries. Common complications of MIGS include transient hyphema, IOP spikes and device-related issues such as malposition or migration.
In addition, MIGS positively impacts patient Satisfaction and QOL. By reducing the anti-glaucoma medications load and controlling IOP, MIGS can lessen the burden of complexities due to difficult medication regimens and their side effects, which leads to better patient satisfaction and treatment adherence.[37,38]
COMPLICATIONS
As MIGS procedures have gained popularity, especially among patients with early glaucoma, it is crucial to address the associated potential complications. Although MIGS generally has a favourable safety profile compared to filtering surgeries, complications can still occur.[24]
In MIGS, transient hyphema is one of the most common complications, which usually resolves on its own within 1–2 weeks. Patients are advised topical steroids to decrease inflammation and fasten recovery. Rarely, persistent or recurrent hyphema may need further intervention, Like an AC washout.[39]
Another frequent issue is an early post-operative IOP fluctuation which is usually due to inflammation, retained viscoelastic material or device obstruction. These IOP spikes are typically temporary and can be managed with anti-glaucoma medications in the early post-operative period. Additional surgical intervention might be required if the IOP spike is severe or persists longer.[40]
Device-related complications may differ based on the particular MIGS performed which might include migration, malposition or obstruction of the device. If a device is malpositioned or has migrated, it may need to be adjusted or removed. In cases where the device is obstructed, treatment options include flushing the device or using medications to lower IOP.[17]
Although very rarely serious complications can also occur following MIGS such as hypotony, choroidal detachment, maculopathy or suprachoroidal haemorrhage. Early hypotony may require observation and anti-inflammatory medications, while in severe cases, surgical intervention is required. Infections like endophthalmitis are rare but can threaten vision.[41] Early diagnosis and prompt treatment with intravitreal antibiotics are essential to prevent permanent loss of vision.
Suprachoroidal haemorrhage is another rare complication which can occur due to excessive intraoperative bleeding. It is usually self-limiting and managed conservatively, but significant or persistent bleeding may require surgical intervention.[33]
Detailed patient evaluation, selection and precise surgical technique are crucial in reducing the risk of complications and maximising the benefits.
Recommendations
MIGS procedures have significantly reduced surgical times by 50% compared to most traditional filtering procedures or GDD implantations. These procedures typically take 15–30 min. The subconjunctival approach seems more effective than other MIGS techniques to achieve pressures in low-teens.
Current evidence confirms that MIGS are effective in reducing IOP and the burden of medications compared to standalone phacoemulsification. Complication rates for MIGS are also less as compared to traditional surgeries. However, for a procedure to be considered clinically advisable, it must be not only safe but also at least as effective as established alternatives. This review reveals a scarcity of studies directly comparing different MIGS techniques or assessing MIGS against pharmacotherapy. More comparative data, especially including standard treatment, could greatly aid ophthalmologists and healthcare authorities in determining the best therapeutic options, potentially reducing medication burdens and associated costs. Significantly more evidence is required to reach this level of certainty. Future research should standardize key outcome features, include comparative treatments and long-term assessments of treatment sustainability and late complications, and preferably be randomised. Reporting washed-out IOPs and incorporating functional and structural markers of glaucoma progression in long-term success definitions are also recommended. In addition, larger cohorts are needed to ensure statistical power and to identify individual biomarkers for truly personalised therapy.
Ultimately, these comprehensive data will equip clinicians with the important details to make evidence-based decisions and select the best option for the management of each glaucoma patient.
CONCLUSION
MIGS has revolutionised the management of glaucoma by providing less invasive and safer alternatives to traditional surgeries. By targeting various anatomical pathways, these devices and procedures improve aqueous humour outflow and reduce IOP. As a result of the diversity of MIGS procedures, including TM bypass stents and subconjunctival and suprachoroidal shunts, each patient can receive treatment tailored for themselves based on disease severity and specific needs.
Although MIGS has shown promising results, long-term outcomes and IOP control must be investigated further. MIGS devices and procedures need comparative studies and randomised controlled trials to determine their relative efficacy and safety in comparison to filtering surgeries. Future research should focus on enhancing current MIGS procedures and exploring novel mechanisms to address the varied causes of glaucoma. This includes refining device designs for better precision and safety and investigating new approaches. In addition, understanding the specific characteristics of different glaucoma subtypes will help tailor treatments more effectively. Identifying which patients are likely to benefit from MIGS.
The advancement of MIGSs represents a transformative change in glaucoma treatment, providing promising prospects for better visual outcomes and QOL. As our knowledge of these surgical techniques, procedures and devices continues to grow, researchers and clinicians are poised to improve patient care further, working toward the critical goal of sight preservation in glaucoma.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch Ophthalmol Chic Ill 1960. 2002;120:1268-79.
- [CrossRef] [PubMed] [Google Scholar]
- Glaucoma treatment trends: A review. Can J Ophthalmol. 2017;52:114-24.
- [CrossRef] [PubMed] [Google Scholar]
- Minimally invasive glaucoma surgery: Current implants and future innovations. Can J Ophthalmol. 2014;49:528-33.
- [CrossRef] [PubMed] [Google Scholar]
- Minimally invasive glaucoma surgery: A critical appraisal of the literature. Annu Rev Vis Sci. 2020;6:47-89.
- [CrossRef] [PubMed] [Google Scholar]
- MIGS and the FDA: What's in a name? Ophthalmology. 2015;122:1737-9.
- [CrossRef] [PubMed] [Google Scholar]
- Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: A systematic review and meta-analysis. PLoS One. 2017;12:e0183142.
- [CrossRef] [PubMed] [Google Scholar]
- Micro-invasive glaucoma surgery: Current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96-104.
- [CrossRef] [PubMed] [Google Scholar]
- Management of complications in glaucoma surgery. Indian J Ophthalmol. 2011;59(Suppl 1):S131-40.
- [CrossRef] [PubMed] [Google Scholar]
- Ab interno trabeculectomy: Development of a novel device (trabectome) and surgery for open-angle glaucoma. J Glaucoma. 2006;15:68-73.
- [CrossRef] [PubMed] [Google Scholar]
- Combined phacoemulsification and microinvasive glaucoma surgery in comparison to phacoemulsification alone for open angle glaucoma. Eye. 2020;34:312-8.
- [CrossRef] [PubMed] [Google Scholar]
- Aqueous outflow-A continuum from trabecular meshwork to episcleral veins. Prog Retin Eye Res. 2017;57:108-33.
- [CrossRef] [PubMed] [Google Scholar]
- Successful reduction of intraocular pressure in a patient with glaucoma secondary to Sturge-Weber syndrome using a suprachoroidal shunt. J Curr Glaucoma Pract. 2020;14:43-6.
- [CrossRef] [PubMed] [Google Scholar]
- Update on minimally invasive glaucoma surgery (MIGS) and new implants. J Ophthalmol. 2013;2013:705915.
- [CrossRef] [PubMed] [Google Scholar]
- iStent inject® W surgery - glaucoma products. Available from: https://www.glaukos.com/glaucoma/products/istent-inject-w [Last accessed on 2024 Apr 08]
- [Google Scholar]
- Initial clinical experience with the trabecular micro-bypass stent in patients with glaucoma. Adv Ther. 2007;24:161-70.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective, unmasked evaluation of the iStent® Inject system for open-angle glaucoma: Synergy trial. Adv Ther. 2014;31:189-201.
- [CrossRef] [PubMed] [Google Scholar]
- iStent trabecular micro-bypass stent for open-angle glaucoma. Clin Ophthalmol Auckl NZ. 2014;8:1937-45.
- [CrossRef] [PubMed] [Google Scholar]
- Micro-invasive glaucoma surgery (MIGS): A review of surgical procedures using stents. Clin Ophthalmol Auckl NZ. 2017;11:1583-600.
- [CrossRef] [PubMed] [Google Scholar]
- Self-expanding nitinol stents: Material and design considerations. Eur Radiol. 2004;14:292-301.
- [CrossRef] [PubMed] [Google Scholar]
- A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: The HORIZON study. Ophthalmology. 2019;126:29-37.
- [CrossRef] [PubMed] [Google Scholar]
- A prospective randomized trial comparing hydrus and iStent microinvasive glaucoma surgery implants for standalone treatment of open-angle glaucoma: The COMPARE study. Ophthalmology. 2020;127:52-61.
- [CrossRef] [PubMed] [Google Scholar]
- Kahook dual blade excisional goniotomy and goniosynechialysis combined with phacoemulsification for angle-closure glaucoma: 6-month results. J Glaucoma. 2019;28:643-6.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of ab-interno irrigating goniectomy with trabectome in primary and secondary glaucoma from a single center in India. Indian J Ophthalmol. 2022;70:3569-74.
- [CrossRef] [PubMed] [Google Scholar]
- Minimally invasive glaucoma surgery (MIGS) In: Albert D, Miller J, Azar D, Young LH, eds. Albert and Jakobiec's principles and practice of ophthalmology. Cham: Springer International Publishing; 2020. p. :1-67.
- [CrossRef] [PubMed] [Google Scholar]
- Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121:855-61.
- [CrossRef] [PubMed] [Google Scholar]
- Gonioscopy-assisted Transluminal Trabeculotomy (GATT) combined phacoemulsification surgery: Outcomes at a 2-year follow-up. Eye. 2023;37:1258-63.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety of the GATT procedure in open-angle glaucoma-6-month results. Int J Environ Res Public Health. 2023;20:2759.
- [CrossRef] [PubMed] [Google Scholar]
- Early outcomes of combined phacoemulsification and Ab interno tanito microhook trabeculotomy in open-angle glaucoma. Ophthalmol Glaucoma. 2024;7:123-30.
- [CrossRef] [PubMed] [Google Scholar]
- Viscodilation of Schlemm's canal for the reduction of IOP via an ab-interno approach. Clin Ophthalmol. 2018;12:2149-55.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and effectiveness of cypass supraciliary micro-stent in primary open-angle Glaucoma: 5-year results from the COMPASS XT study. Am J Ophthalmol. 2019;208:219-25.
- [CrossRef] [PubMed] [Google Scholar]
- Alcon announces voluntary global market withdrawal of CyPass Micro-Stent for surgical glaucoma. 2018. Alcon.com. Available from: https://www.alcon.com/media-release/alcon-announces-voluntary-global-market-withdrawal-cypass-micro-stent-surgical [Last accessed on 2024 Feb 15]
- [Google Scholar]
- iStent supra micro-bypass stent - Beye. Available from: https://www.beye.com/r/p/111 [Last accessed on 2024 Feb 16]
- [Google Scholar]
- Suprachoroidal bleeding after XEN gel implantation. J Glaucoma. 2017;26:e261-3.
- [CrossRef] [PubMed] [Google Scholar]
- What is the XEN® gel stent? - XEN® Gel Stent. Available from: https://www.xengelstent.com/XENGelStent [Last accessed on 2024 Feb 16]
- [Google Scholar]
- Three-year follow-up of a novel aqueous humor MicroShunt. J Glaucoma. 2016;25:e58-65.
- [CrossRef] [PubMed] [Google Scholar]
- The basics of good postoperative care after glaucoma surgery. Community Eye Health. 2016;29:29-31.
- [Google Scholar]
- The case for standalone micro-invasive glaucoma surgery: Rethinking the role of surgery in the glaucoma treatment paradigm. Curr Opin Ophthalmol. 2023;34:138-45.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life after combined cataract and minimally invasive glaucoma surgery in glaucoma patients. Clin Ophthalmol. 2020;14:3049-56.
- [CrossRef] [PubMed] [Google Scholar]
- Microinvasive glaucoma surgery: A review of 3476 eyes. Surv Ophthalmol. 2021;66:714-42.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of microinvasive glaucoma surgery. J Ophthalmol. 2017;2017:3182935.
- [CrossRef] [PubMed] [Google Scholar]
- Late-onset endophthalmitis after XEN45® implantation: A retrospective case series and literature review. J Curr Glaucoma Pract. 2022;15:153-60.
- [CrossRef] [PubMed] [Google Scholar]






