Translate this page into:
Pre-operative optical coherence tomography macula: An indispensable investigation to predict post-operative outcomes
*Corresponding author: Sivaranjani Balraj, Department of Ophthalmology, Government Medical College, Surat, Gujarat, India. ranjani.balraj@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Solu TM, Balraj S, Patel I, Acharya S. Pre-operative optical coherence tomography macula: An indispensable investigation to predict post-operative outcomes. Glob J Cataract Surg Res Ophthalmol 2022;1:59-63.
Abstract
Objectives:
The aim of the study was to identify clinically undiagnosed macular pathologies and determine their distribution in pre-operative cataract patients.
Materials and Methods:
The present study is a hospital-based cross-sectional study of patients who attended the ophthalmology OPD to undergo pre-operative evaluation for cataract surgery in a tertiary care government hospital in South Gujarat, India, during a period from July 2019 to September 2020. The inclusion criteria for the study were patients above the age of 40 years with immature cataracts that allowed for optical coherence tomography (OCT) scan acquisition. Patients with prior history of macular disease or those who have undergone treatment for retinal pathologies were excluded from the study. Two hundred cataract patients who met the defined inclusion criteria and gave an informed written consent were enrolled. Three hundred and ten eyes with a normal fundoscopic appearance underwent spectral domain OCT macula examination before cataract surgery. The OCT macula scans were scrutinised for any undiagnosed subtle macular pathologies and were documented. Cataract surgery with explained visual prognosis was undertaken in specific cases correlating with their macular findings and surgery was deferred in a few.
Results:
Among 200 study participants, 310 eyes had unremarkable fundoscopic appearance on ophthalmoscopy. While undergoing OCT examination, 65 (20.96%) eyes out of 310 showed abnormalities. The most common presentation was epiretinal membrane in 22 eyes (7.09%) followed by pigment epithelial detachment in 14 (4.5%) eyes. Other findings were drusen (nine eyes), lamellar macular hole (five), vitreomacular traction (five), IS-OS junction abnormalities (four), retinal pigment epithelium alterations (two), juxtafoveal telangiectasia (one), foveal thinning (one), pseudovitelliform lesion (one) and spongy oedema (one).
Conclusion:
Routine fundoscopic examination for pre-operative cataract evaluation of the 200 study participants failed to identify subtle macular pathologies which were further diagnosed on SD-OCT Macula. Sixty-five eyes (20.96%) out of the 310 eyes were found to have macular pathologies. The various findings that were obtained provided us an insight into the appropriate choice of intraocular lenses, required surgical consents and the therapeutic strategy for each individual patient. Thus, we can conclude that OCT macula is an indispensable investigation before cataract extraction to avoid unsolicited post-operative surprises and medicolegal conundrums.
Keywords
Spectral domain optical coherence tomography macula
Cataract surgery
Occult macular pathologies
INTRODUCTION
Cataract is the leading cause of blindness in the world with the World Health Organization estimating that nearly 17.7 million suffer from cataract and the number only keeps increasing as the world population grows.[1]
Cataract surgery has been revolutionised with the introduction of phacoemulsification, femtosecond laser-assisted cataract surgery,[2] correction of corneal astigmatism and the use of premium intraocular lenses[3] to get the best post-operative visual outcomes.
Often due to media opacities and inadequate pupil dilatation for detailed evaluation, subtle macular changes cannot be identified on routine fundoscopy. These undiagnosed macular pathologies may result in erroneous treatment decisions, leading to suboptimal visual outcomes. Hence, identifying these subtle changes become of prime importance to the operating surgeon and aid in managing the modern-day cataract patient’s expectations.
Optical coherence tomography (OCT) is a non-invasive imaging technique which utilises Michelson’s interferometry principle. It is an optical analogue of ultrasound imaging that uses low coherence interferometry to produce cross-sectional images of the retina[4] and is a critical tool in the diagnosis and monitoring of ocular disease involving the retina, choroid and optic nerve.[5] OCT permits evaluation of tissue pathology at the cellular level, achieving a resolution of 2–3 μm and can image through most media opacities including vitreous haemorrhage, cataract and silicone oil.[6]
In spectral domain (SD) OCT, the light composing the interference spectrum of echo time delays is measured simultaneously by a spectrometer and a high-speed charge-coupled device, thereby the information of the full depth scan can be acquired within a single exposure. SD OCT’s higher acquisition speeds allow for a shift from two-dimensional to three-dimensional (3D) images of ocular anatomy.[5]
MATERIALS AND METHODS
The required permissions to conduct a hospital-based cross-sectional study were obtained from the Institute’s Human Research Ethics Committee before the commencement of the study.
Patients visiting the ophthalmology OPD to undergo pre-operative evaluation for cataract surgery in a tertiary care government hospital in South Gujarat, India, and consenting to be subjects of the study were enrolled. They were evaluated during a period from July 2019 to September 2020 and were examined thoroughly as per the protocol.
Patients included in the study were individuals aged above 40 years of age with immature cataracts, a normal appearing macula on fundoscopy and giving informed written consent to be a part of the study.
Patients with mature and dense posterior subcapsular cataracts which preclude capturing of OCT images, preexisting retinal or macular pathologies and patients who have already undergone treatment for retinal disorders (intravitreal injections/vitreoretinal surgeries/LASER treatment) were excluded from the study.
The participating patients underwent establishment of best-corrected visual acuity by an illuminated Snellen’s chart at 6 m distance and near vision measured with Roman’s near vision chart, anterior segment examination and cataract grading using slit lamp, dilated (with 0.5% tropicamide) posterior segment examination using direct and indirect ophthalmoscope (Heine Omega 500 LED, Munich, Germany), slit-lamp biomicroscopy of the fundus using + 78D lens (Volk double aspheric lens, USA). Fundoscopy in the OPD was carried out by consultant general ophthalmologists.
Patients with a normal fundoscopic appearance on biomicroscopy underwent OCT Macula by SD OCT using Topcon 3D OCT-1 Maestro (Topcon Europe, Netherlands).
The 3D macula protocol was used on OCT for macular thickness measurements. It consists of a raster scan composed of 256 × 256 (vertical × horizontal) axial scans covering an area of 6×6 mm2 in the macular region. It identifies the layers of the retina and determines macular thickness by measuring the distance between the inner limiting membrane and the inner boundary of retinal pigment epithelium (RPE) in each of the nine regions.[7]
Statistical analysis
Descriptive statistical analysis has been carried out in the present study. Results on continuous measurements are presented as mean ± SD (min-max) and results on categorical measurements are presented in numbers and percentages.
Statistical analysis was done using OpenEpi version 3.01 (online) updated April 06, 2013. P < 0.05 was considered significant. OpenEpi is a free and open browser software for epidemiological statistics by Rollins School of Public Health, USA.
RESULTS
Three hundred and ten eyes of the 200 study participants were included in the study. The mean age of the 200 study participants was found to be 60.6 ± 9 years of age, with the minimum age being 40 years and maximum being 85 years of age. The maximum number of patients was in the age group of 60–69 years and the least number of patients were in the age group of ≥80 years. Among them, 107 (53.5%) were male and 93 (46.5%) were female [Chart 1].

- Age-wise distribution of the study participants.
In the present study, 245 eyes were labelled as normal on clinical examination which was found to be consistent with the OCT findings. Fundoscopy failed to identify subtle macular pathologies in 65 eyes.
Among the identified pathologies, the most common finding was the presence of an epiretinal membrane (ERM) which was found in 22 eyes. Other OCT findings were pigment epithelial detachment [Figure 1] (n = 14), drusen (n = 9), lamellar macular hole (n = 5), vitreomacular traction [Figure 2] (n = 5), IS-OS junction abnormalities (n = 4), RPE alterations (n = 2), juxtafoveal telangiectasia (n = 1), foveal thinning (n = 1), pseudovitelliform lesion (n = 1) and spongy oedema (n = 1) [Chart 2 and Table 1].

- Optical coherence tomography showing pigment epithelial detachment.

- Optical coherence tomography showing focal vitreomacular traction due to ERM.
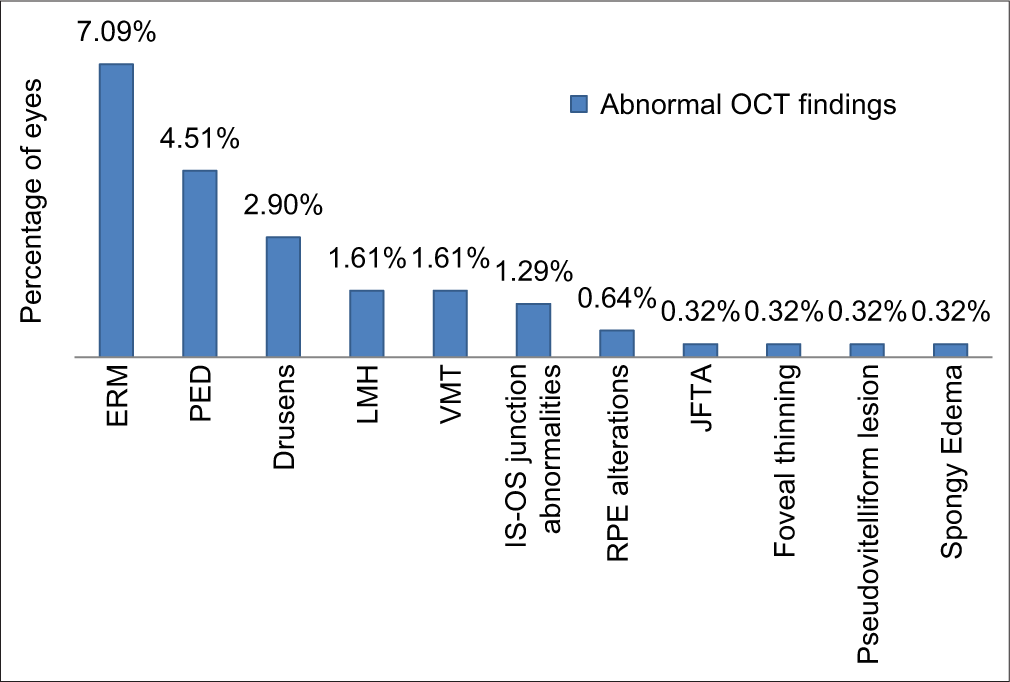
- Distribution of abnormal optical coherence tomography findings.
| OCT findings | No. of eyes | Percentage (out of 310 eyes) (%) |
|---|---|---|
| Epiretinal membrane | 22 | 7.09 |
| Pigment epithelial detachment | 14 | 4.51 |
| Drusen | 9 | 2.90 |
| Lamellar macular hole | 5 | 1.61 |
| Vitreomacular traction | 5 | 1.61 |
| IS–OS junction abnormalities | 4 | 1.29 |
| Retinal pigment epithelium alterations | 2 | 0.64 |
| Juxtafoveal telangiectasia | 1 | 0.32 |
| Foveal thinning | 1 | 0.32 |
| Pseudovitelliform lesion | 1 | 0.32 |
| Spongy oedema | 1 | 0.32 |
| Total | 65 | 20.96 |
Drusen were identified in 9 (2.90%) eyes. Of these, two eyes had subfoveal drusen and one eye had a juxtafoveal drusen. The previous studies have found that patients with drusen have a higher risk of developing exudative AMD,[8] thus the diagnosis of drusen identifies such patients at risk.
DISCUSSION
Based on defined inclusion and exclusion criteria, 200 patients undergoing routine pre-operative evaluation for cataract surgery in a tertiary care hospital in South Gujarat between July 2019 and September 2020 were enrolled in our study. Three hundred and ten eyes had normal fundoscopy findings on fundus biomicroscopy.
Sixty-five (20.96%) eyes were diagnosed with abnormalities on OCT macula scans which were undiagnosed on routine fundoscopy.
In Neto et al.[9] study conducted in 2015, 98 eyes of 98 patients were evaluated and underwent SD-OCT 5 h before surgery. OCT preoperatively diagnosed macular disease in 21 eyes (21.4%). This value was found comparable to our study.
In Weill et al.[10] study conducted in 2018, 453 eyes of 453 patients were enrolled in the study and underwent SDOCT macula. Among these eyes, 22.8% of them were found to have macular pathologies which were previously missed on fundoscopy.
In Huang et al. study[11] conducted in 2017, 1176 eyes of 1176 patients underwent a pre-operative OCT macula. One hundred and eighty-four eyes were excluded from the study due to poor scan quality. Among them, 294 (25%) eyes showed macular pathologies.
In Kowallick et al.[12] study conducted in 2018, 174 eyes of 133 patients were enrolled in the study. Twelve eyes of 10 patients were excluded from the study due to poor OCT scan quality. One hundred and sixty-two eyes underwent successful SD-OCT macula. Among these, 20 eyes (12.35%) had pathologies with potential impact on visual outcome. They also classified 69 eyes (42.59%) as eyes with degenerative vitreous changes without any impact on visual outcome.
In Zafar et al.[13] study conducted in 2016, 155 eyes of 155 patients were included in the study and underwent SSOCT, of these 17 (10.9%) eyes showed macular pathologies.
In Klein et al.[14] study conducted in 2016, 265 eyes of 149 patients were evaluated and underwent SD-OCT macula. Among these, 35 (13.2%) eyes showed macular abnormalities.
In Sudhalkar et al.[15] study conducted in 2018, 1539 eyes were enrolled in the study. Seventy-five eyes (4.87%) precluded OCT scan imaging and were excluded from the study. Out of the 1444 eyes, 133 (9.2%) eyes showed macular abnormalities.
In the present study, out of the 310 eyes evaluated with OCT macula, 22 (7.09%) eyes showed an ERM. One eye showed an ERM with traction resulting in a lamellar macular hole (ERM with LMH). One eye showed an ERM in the presence of subretinal fluid.
In Kowallick et al.[12] study, out of the 162 eyes studied, 10 (6.17%) eyes showed an ERM, two of these caused foveal elevation due to traction. These findings are comparable to our study.
In Huang et al.[12] study, out of 1176 eyes studied, 130 (11.05%) showed an ERM on OCT macula. This can be attributed to its larger sample size.[15]
In Neto et al.[9] study, out of the 98 eyes included in the study, 3 (3.06%) eyes had an ERM on OCT macula.
In Zafar et al.[13] study, out of the 155 eyes studied, 4 (2.58%) eyes showed an ERM on SS-OCT Macula.
In Klein et al.[14] study, 265 eyes were enrolled in the study and 11 (4.15%) eyes showed an ERM which was identified on OCT macula.
In Sudhalkar et al.[15] study, out of the 1444 eyes that underwent successful OCT scans, 53 (3.67%) eyes showed an ERM.
An ERM is slowly progressive and leads to visual impairment only in some cases.[16] It has been demonstrated that patients with a pre-operative ERM have higher risk for the development of post-operative pseudophakic cystoid macular oedema, epimacular membrane rip and potentially lead to visual impairment.[17]
Drusen were identified in 9 (2.90%) eyes. Of these, two eyes had subfoveal drusen [Figure 3] and one eye had a juxtafoveal drusen.

- Optical coherence tomography showing multiple subfoveal drusen.
In Kowallick et al.[12] study, SD-OCT imaging revealed drusen in 4 (2.47%) eyes, of these two eyes had subfoveal drusen. This was found comparable with our study.
In Klein et al.[14] study, 15 (5.66%) eyes showed AMD, this could be attributed to the higher number of older individuals in their study.
In Huang et al.[11] study, 10 (0.85%) eyes of the 1176 eyes studied showed age-related macular degeneration.
The previous studies have found that patients with drusen have a higher risk of developing exudative AMD,[18] thus the diagnosis of drusen identifies such patients at risk.
The limitation of our study is the absence of post-operative follow-up examination. Accordingly, we have not been able to identify the post-operative consequences of the macular pathologies and its correlations with the visual outcome. Therefore, it would be more beneficial to conduct a prospective interventional study comparing the functional outcomes with pre-operative OCT macula.
CONCLUSION
We were able to diagnose subtle macular pathologies which went undiagnosed on routine fundoscopy done during pre-operative evaluation of cataract in a significant (20.96%) number of patients on SD-OCT macula which could have had a potential impact on the post-operative visual outcomes. It might be important to know if these subtle retinal findings would have had any impact on the visual outcome.
These were conveyed to the primary treating surgeon. Accordingly, their surgery was deferred or cataract extraction was done by experienced senior surgeons with explained visual prognosis.
By incorporating SD-OCT macula in routine pre-operative evaluation of patients for cataract surgery, we can also avoid medicolegal implications that might arise from postoperative patient dissatisfaction.
Therefore, these subtle macular pathologies should be diagnosed preoperatively so as to aid us in deciding the therapeutic strategy, to improve patient’s informed consent and to estimate the visual expectancy.
Thus, we conclude that pre-operative OCT macula is of paramount importance to identify occult macular pathologies and should be used as a mandatory screening tool in patients posted for cataract surgery.
The routine use of OCT before cataract surgery will enable ophthalmologists to make relevant therapeutic decisions, appropriate choice of IOLs, modify the informed consent and temper down patient’s post-operative expectations accordingly.
In today’s era, where cataract surgery is a refractive as well as a rehabilitative procedure, it becomes imperative to make OCT a mandatory tool in the pre-operative evaluation of cataract patients to screen for occult macular disease.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The prevalence and risk factors for cataract in rural and urban India. Indian J Ophthalmol. 2019;67:477.
- [CrossRef] [PubMed] [Google Scholar]
- National Programme for Control of Blindness and Visual Impairement. New Delhi: Dr. Rajendra Prasad Centre for Ophthalmic Sciences; 2019. p. :18.
- [Google Scholar]
- Clinical outcomes of femtosecond laser-assisted cataract surgery versus conventional phacoemulsification surgery for hard nuclear cataracts. J Cataract Refract Surg. 2017;43:486-91.
- [CrossRef] [PubMed] [Google Scholar]
- Optical coherence tomography: A guide to interpretation of common macular diseases. Indian J Ophthalmol. 2018;66:20.
- [CrossRef] [PubMed] [Google Scholar]
- Optical coherence tomography In: Yanoff M, Duker JS, eds. Ophthalmology (4th ed). China: Elsevier Saunders; 2014. p. :448.
- [Google Scholar]
- Assessment of the effect of age on macular layer thickness in a healthy Chinese cohort using spectral-domain optical coherence tomography. BMC Ophthalmol. 2018;18:169.
- [CrossRef] [PubMed] [Google Scholar]
- The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: The beaver dam eye study. Ophthalmology. 2015;122:787-95.
- [CrossRef] [PubMed] [Google Scholar]
- Optical coherence tomography in patients undergoing cataract surgery. Arq Bras Oftalmol. 2015;78:241-5.
- [CrossRef] [PubMed] [Google Scholar]
- Patient management modifications in cataract surgery candidates following incorporation of routine preoperative macular optical coherence tomography. J Cataract Refract Surg. 2021;47:78-82.
- [CrossRef] [PubMed] [Google Scholar]
- Macular assessment of preoperative optical coherence tomography in ageing Chinese undergoing routine cataract surgery. Sci Rep. 2018;8:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Optical coherence tomography findings in patients prior to cataract surgery regarded as unremarkable with ophthalmoscopy. PLoS One. 2018;13:e0208980.
- [CrossRef] [PubMed] [Google Scholar]
- Swept-source optical coherence tomography to screen for macular pathology in eyes having routine cataract surgery. J Cataract Refract Surg. 2017;43:324-7.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative macular spectral-domain optical coherence tomography in patients considering advanced-technology intraocular lenses for cataract surgery. J Cataract Refract Surg. 2016;42:537-41.
- [CrossRef] [PubMed] [Google Scholar]
- Incorporating optical coherence tomography in the cataract preoperative armamentarium: Additional need or additional burden? Am J Ophthalmol. 2019;198:209-14.
- [CrossRef] [PubMed] [Google Scholar]
- The incidence of idiopathic preretinal macular gliosis. Ann Ophthalmol. 1985;17:378-80.
- [Google Scholar]
- Preexisting epiretinal membrane is associated with pseudophakic cystoid macular edema. Graefe's Arch Clin Exp Ophthalmol. 2018;256:909-17.
- [CrossRef] [PubMed] [Google Scholar]
- Subjective and functional deterioration in recurrences of neovascular AMD are often preceded by morphologic changes in optic coherence tomography. Br J Ophthalmol. 2011;95:1424-6.
- [CrossRef] [PubMed] [Google Scholar]






