Translate this page into:
Secondary piggyback intraocular lens for posterior microphthalmos
*Corresponding author: Vitor Miranda, Department of Ophthalmology, Centro Hospitalar de Entre o Douro e Vouga, Santa Maria da Feira, Portugal. vitormiranda94@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Miranda V, Aguiar C, Ambrósio J, Gomes da Rocha A, Chibante Pedro J. Secondary piggyback intraocular lens for posterior microphthalmos. Glob J Cataract Surg Res Ophthalmol. 2023;2:73-8. doi: 10.25259/GJCSRO_17_2023
Abstract
We describe a rare case of posterior microphthalmos (PM) submitted to cataract surgery and highlight the problem of high residual hyperopia in these eyes due to the limited commercial availability of a high-powered intraocular lens (IOL). A 50-year-old highly hyperopic man who pretended refractive surgery presented in our hospital. Pre-operative Snellen best-corrected visual acuity (BCVA) was 20/125 in the right eye with a spherical correction of +17.00 D and 20/40 in the left eye with a spherical correction of +18.75. The anterior segment and fundus exam were typical for PM. Optical axial length was 15.50 mm in the right eye and 15.53 mm in the left eye and anterior segment dimensions were within normal limits. IOL power calculation for emmetropia ranged between +51.5 and +62.5 D using modern IOL formulas. Cataract surgery was performed in a bilateral and sequential manner with the implantation of monofocal foldable acrylic +45.0 D IOL with the improvement of spherical correction to +11.00 D bilaterally. Secondary implantation of supplementary sulcus IOL with the maximum +10.0 D power was then performed bilaterally and sequentially with a final BCVA of 20/125 in the right eye with spherical correction of +5.75 D and 20/40 in the left eye with spherical correction of +5.25 D. Piggyback IOL implantation is beneficial and, in most times, necessary in PM since one IOL will not be sufficient to achieve emmetropia. Different piggyback strategies in regard to timing (primary vs. secondary) and chosen IOLs (in-the-bag, sulcus, and iris-claw) can be used.
Keywords
Posterior microphthalmos
Microphthalmos
Piggyback intraocular lens
Cataract surgery
Refractive surgery
INTRODUCTION
Microphthalmos is usually defined by a short axial length (AL), typically 2 standard deviations below the mean for age, corresponding to an AL below 21 mm in adult eyes. This rare condition has an estimated prevalence ranging from 0.2 to 19.0/10,000, although there is some heterogeneity in its definition.[1] Simple microphthalmos is defined by the short AL and high hyperopia in the absence of anatomical malformations, whereas complex microphthalmos is also accompanied by gross anatomical malformations such as anterior segment dysgenesis, chorioretinal colobomas or dysplastic retina.
Within simple microphthalmos, there are two distinct phenotypes: nanophthalmos and posterior microphthalmos (PM). While nanophthalmos is characterised by a proportionally small anterior segment (in relation to the short AL), in PM the anterior segment dimensions are relatively normal, with a normal corneal diameter, anterior chamber depth, and lens/eyeball volume ratio.[2] Due to this difference, the incidence of angle-closure glaucoma is markedly different between these groups, with Relhan et al. having described an incidence of 69.2% in nanophthalmos versus 0% in PM.[3] Other common characteristics of PM are papillomacular folds and/or wrinkles with absent foveal depression, chorioretinal folds, crowded discs resembling papilledema (pseudopapilledema), sclerochoroidal thickening, uveal effusions, and high corneal power.[4] In addition, high hyperopia and short AL may cause esotropia, increasing the risk of amblyopia in patients with PM.
Cataract surgery in microphthalmos presents several challenges, including uveal effusion, aqueous misdirection, choroidal haemorrhage, cystoid macular oedema, and the decreased accuracy of standard methods for intraocular lens (IOL) power calculation.[5] At present, there is no consensus on the best formula for calculating lens power in small eyes, but some reports suggest that Hoffer Q, Holladay II, and Haigis may perform better.[6] In other reports, Kane and SRK/T formulas outperformed Hoffer Q and Barrett Universal II in short eyes.[5] Not only are the formulas less accurate, but also the calculated IOL power is often very high and with limited commercial availability. In PM, this problem is even more exacerbated than in nanophthalmos, since these eyes have a ‘disproportionately large’ anterior segment in regard to the AL, which leads to a very posterior effective lens position and the need for very high dioptric power to achieve emmetropia, which might be insufficiently corrected with only one IOL.
To address this problem, several strategies have been reported, such as (1) primary piggyback IOL implantation with placement of one (or even two) IOLs in the capsular bag and another in the ciliary sulcus, (2) secondary piggyback IOL implantation in the ciliary sulcus or (3) secondary iris-claw IOL implantation.[7-9] Previous research has indicated that laser refractive surgery for correcting hyperopia may not be suitable for microphthalmos patients, as it is difficult to achieve reliable and predictable results in cases of extreme refractive errors through methods such as laser in situ keratomileusis and photorefractive keratectomy.[10]
We present a case of PM treated with bilateral cataract surgery with implantation of a high-powered IOL and secondary ciliary sulcus IOL implantation.
CASE REPORT
A 50-year-old highly hyperopic man, with a history of amblyopia in the right eye due to infantile esotropia, presented in our hospital asking for refractive surgery. He had no ocular surgical history and no other significant medical history. Pre-operative Snellen best-corrected visual acuity (BCVA) was 20/125 in the RE with +17.00 spherical correction and 20/40 in the left eye with +18.75 spherical correction. The patient did not use any prismatic correction. Intraocular pressure was 18 mmHg in both eyes. Anterior segment exam was unremarkable, with a clear cornea, normal anterior chamber depth, and lens with very subtle nuclear sclerosis. Fundus exam revealed crowded optic discs with papillomacular folds and otherwise macular and retinal vascular patterns within normal limits.
Macular optical coherence tomography (OCT) confirmed papillomacular folds with the absence of foveal depression, choroidal thickening, and a crowded disc without cupping [Figure 1].
The patient had previously tried contact lens for 2 times in his adult life but had never been fully satisfied with them and abandoned these options due to poor comfort and dry eye symptoms so a surgical option was considered.
Optical biometry with the AL-Scan® Optical Biometer (NIDEK) showed an AL of 15.50 mm in the right eye and 15.53 mm in the left eye [Figure 2]. Other biometric measurements are compiled in [Table 1].

- Fundus Spectralis® Multicolor imaging of of both eyes with crowded and cupless disc (above). Macular optical coherence tomography (OCT) scans passing through the fovea with papillomacular folds with absent foveal depression (down). Left eye optic disc OCT with crowded optic disc.
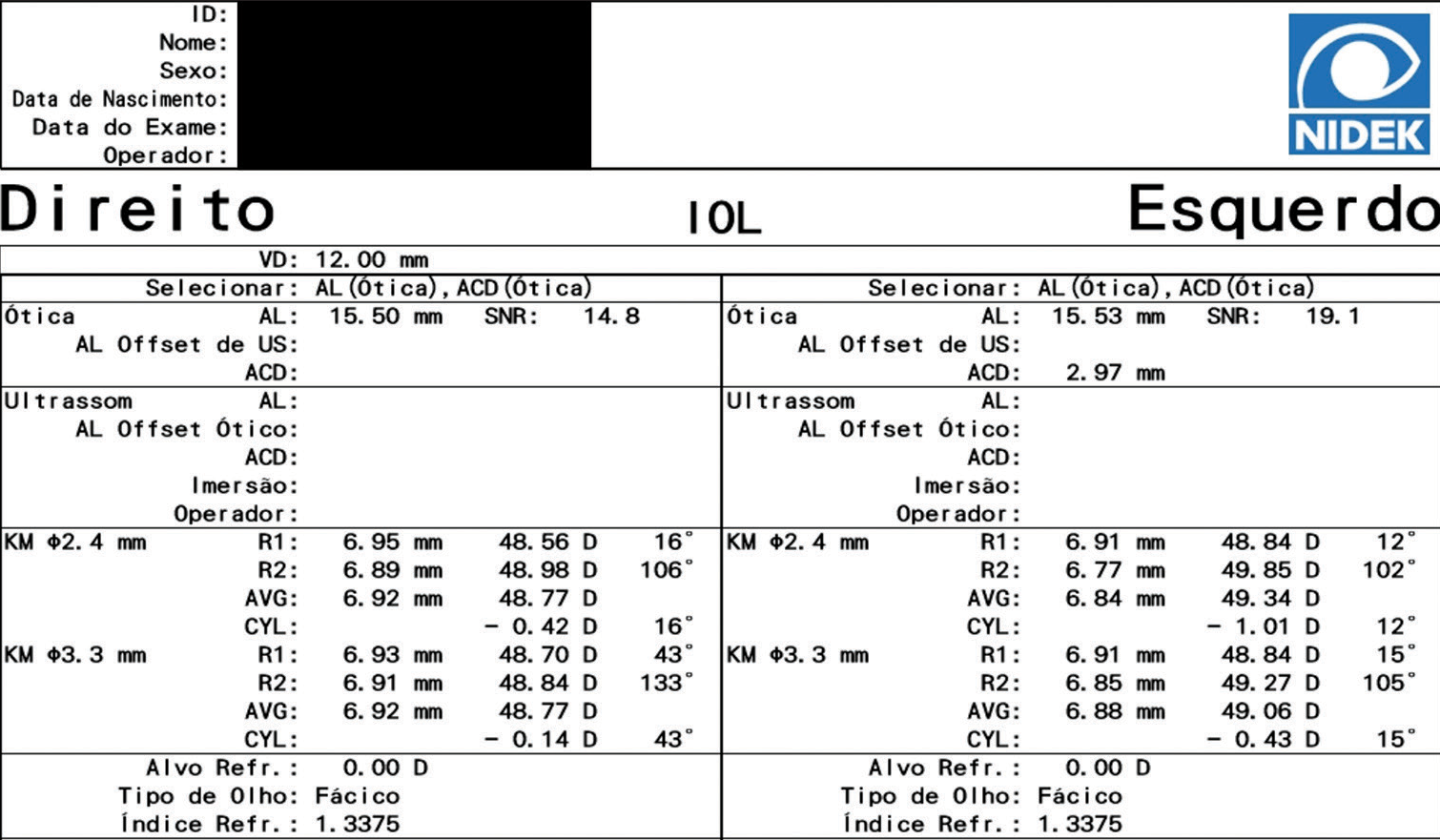
- Printout from the optical biometer (AL-Scan, NIDEK). IOL: Intraocular lens, AL: Axial length, ACD: Anterior chamber depth, SNR: Signal-to-noise ratio, US: Ultrasound, KM: Keratometry, AVG: Average, CYL: Cylinder, D: Diopter
| Right eye | Left eye | |
|---|---|---|
| Axial length (AL-Scan®Optical Biometer [NIDEK]) | 15.50 mm | 15.53 mm |
| Keratometry (central 2.4 mm) (AL-Scan® Optical Biometer [NIDEK]) |
||
| Steep | 48.98 D | 49.85 D |
| Flat | 48.56 D | 48.84 D |
| Average | 48.77 D | 49.34 D |
| Astigmatism | 0.41 D | 1.01 D |
| Central corneal thickness (EM-3000®[Tomey]) |
612 µm | 606 µm |
| Endothelial cell count (EM-3000®[Tomey]) |
2487 | 3097 |
| Anterior chamber depth (Pentacam®AXL Wave [Oculus]) |
3.37 mm | 3.21 mm |
| Horizontal white-to-white (Pentacam®AXL Wave [Oculus]) |
11.2 mm | 11.0 mm |
IOL power with a Plano target was calculated with different traditional and new generation formulas. As the highest available power for monofocal foldable acrylic (TECNIS® ZCB00, Johnson and Johnson) IOL in stock at our institution was +34.0 D, a special request was made for a higher powered monofocal foldable acrylic IOL within the commercially available market in our country. The selected IOL was a monofocal foldable acrylic IOL (CT Spheris 204, Zeiss) which is available with powers from +31.0 D to +45.0 D on request. Several of newer generation formulas could not be used due to a shorter than accepted axial length. With the formulas that could be used, the calculated dioptric power necessary for emmetropia ranged between +51.5 D and +62.5 D for the right eye and between +50.5 D and +61.5 D for the left eye. IOL power calculations with the different formulas are shown in [Table 2].
| Right eye | Left eye | |
|---|---|---|
| SRK/T (1) | +51.5 D | +50.5 D |
| Hoffer® QST (2) | +53.0 D | +52.5 D |
| Hoffer Q (1) | +62.0 D | +61.0 D |
| Haigis (1) | +62.5 D | +61.0 D |
| LADAS super formula (3) | +62.5 D | +61.5 D |
| EVO, Hill-BRF, Hoffer, Kane, Pearl DGS, Barret II (4) | X | X |
(1) Traditional formulas were calculated in the online calculator https://iolzero.com/. (2) Hoffer® QST was calculated in the available website https://hofferqst.com/. (3) The LADAS super formula was calculated using the developer website https://www.iolcalc.com/. (4) Several newer generation formulas available in https://iolcalculator.escrs.org/, could not be used for parameters (axial length) falling outside the accepted range
As the highest available power for in-the-bag IOL was the +45.0 D IOL from Zeiss (CT Spheris 204), it was decided to implant this IOL bilaterally and afterward consider a secondary implant. The patient was informed of the risk of cataract surgery in microphthalmos and the refractive strategy planned and informed consent was obtained. Bilateral, sequential cataract surgery with implantation of +45 D IOL was done without complications. A sutureless approach with a 2.4 mm incision was used and pre-operative mannitol was used to dehydrate the vitreous and minimise posterior pressure. Post-operative drops were used, namely a combination of dexamethasone phosphate 1 mg/mL plus ofloxacin 3 mg/mL 5 times a day for 2 weeks and another drop with tromethamine ketorolac 5 mg/mL 3 times a day for 1 month.
Nonetheless, the patient remained highly hyperopic, with a BCVA of 20/125 in the right eye and 20/40 in the left eye with spherical correction of +11.0 D in both eyes.
It was then decided to implant a supplementary IOL in the ciliary sulcus (Sulcoflex Aspheric®, Rayner) with the highest available power provided by the manufacturer. Bilateral, sequential, implantation of sulcus +10.0 D IOL was done without complications after 6 months of the primary surgery for both eyes [Figure 3], with a final BCVA of 20/125 in the right eye with spherical correction of +5.75 D and 20/40 in the left eye with spherical correction of +5.25 D after 1 month of each surgery.
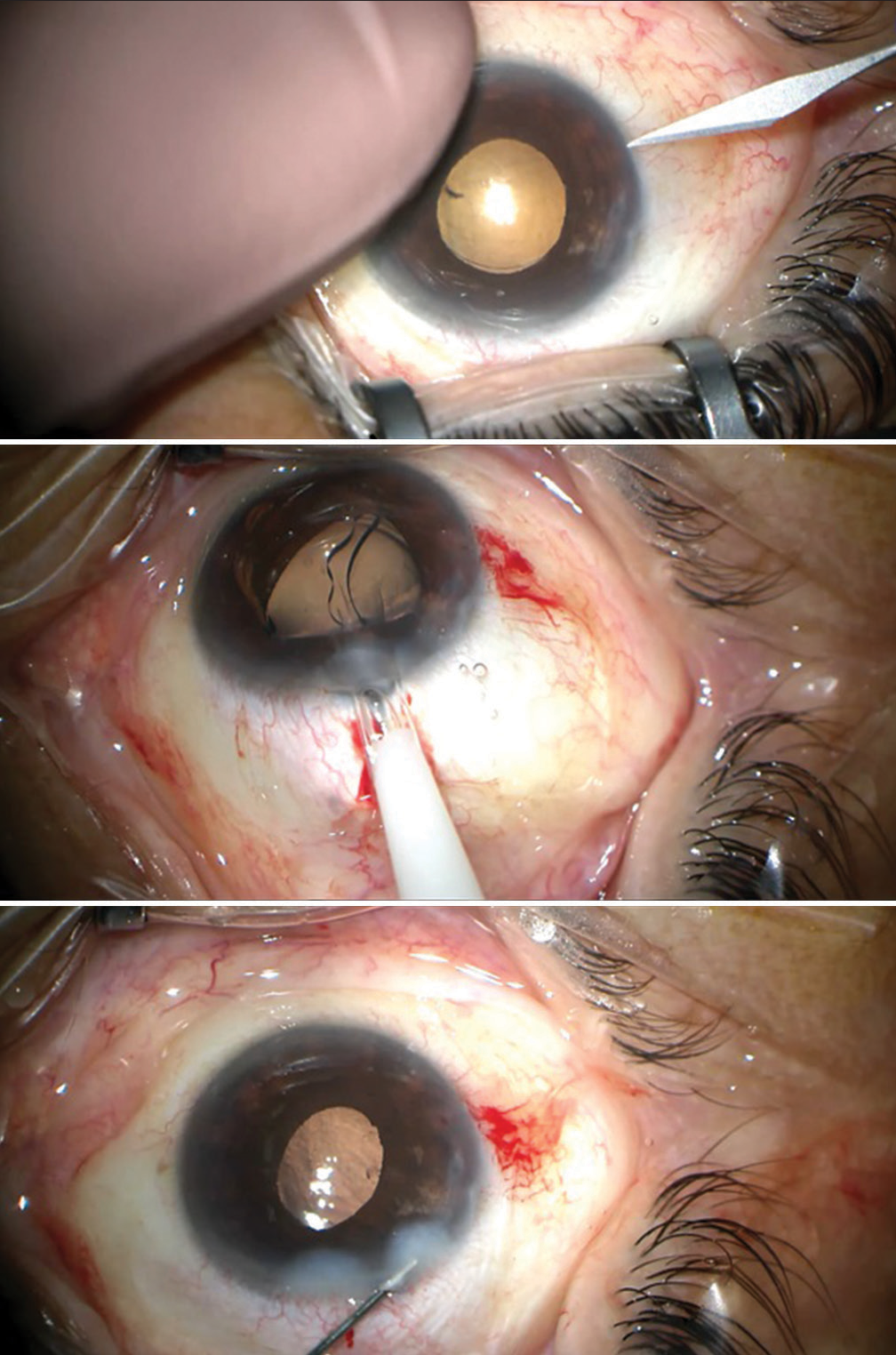
- Intra-operative images of implantation of Sulcoflex intraocular lens. Notice the redundant conjunctiva in the superior and inferior fornix in this extremely short eye.
At 5 months postoperatively, BCVA remained unchanged and the anterior segment exam was unremarkable with normal intraocular pressure. There were no signs of interlenticular opacification (ILO), angle closure, uveitis, or pigment dispersion from iris chafing. Anterior segment OCT showed the correct placement of the sulcus IOL with normal angle morphology and maintained anterior chamber depth [Figure 4]. Post-operative fundus dilated exam and macular OCT were unremarkable, apart from the changes already described preoperatively. Despite significant residual hyperopia, the patient was reasonably satisfied with the refractive outcome since his reduced thickness glasses were now more aesthetically pleasing and he reported he could now even perform some activities of daily living independent of glasses. Because of this, the patient did not want to pursue further refractive surgery for the time being.
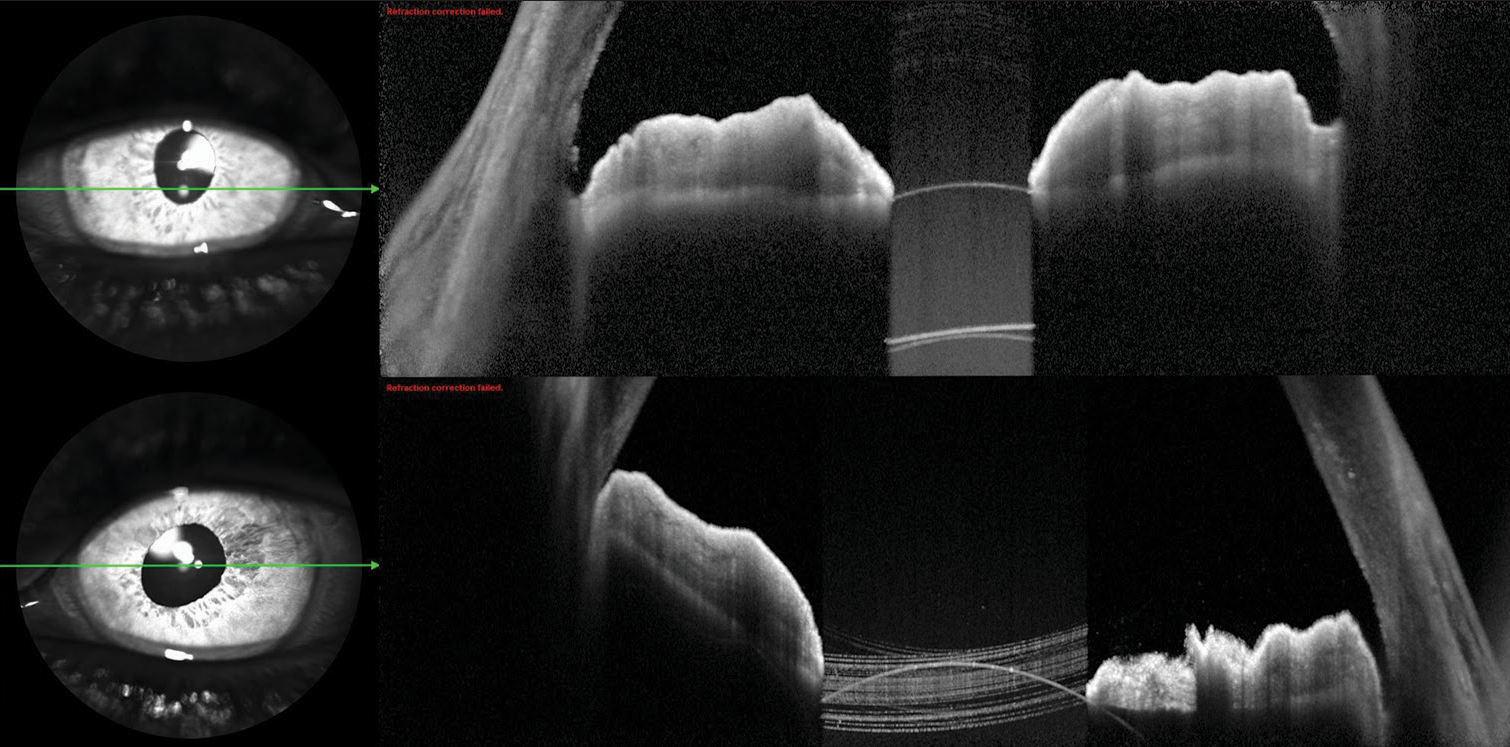
- Anterior segment optical coherence tomography (Heidelberg) images after implantation of a supplementary intraocular lens in both eyes. The anterior chamber depth and angle morphology are maintained.
DISCUSSION AND CONCLUSION
Due to the limited availability of foldable IOLs with power above 35 D, eyes with PM are often left with high residual hyperopia after lens extraction or cataract surgery. Several strategies have been reported to deal with this high residual hyperopia, each with its shortcomings.
Secondary piggyback IOL implantation (whether in the sulcus or iris-claw) is probably beneficial over primary piggyback since IOL power calculation for the second IOL can be based on actual post-operative refraction instead of unreliable predictions, although a second surgery is required.
Implantation of more than one IOL in the capsular bag can lead to a late complication known as ILO. This complication can happen months to years after the procedure and is caused by the build-up of acellular material and or/lens epithelial cell growth and can lead to impaired visual acuity and hyperopic shift. However, this problem can be minimised with the implantation of two different types of IOLs or by placing one in the bag and another in the sulcus.[7-9,11] In our particular case, a hydrophilic IOL with a hydrophobic surface was implanted in the bag (Zeiss CT Spheris) and a hydrophilic IOL (Rayner Sulcoflex Aspheric) was implanted in the sulcus.
Iris-claw lens implantation (Artisan® Aphakia, Ophtec) is another option in these eyes, although these lenses have a minimum requirement of 2.8 mm anterior chamber depth and should not be used in patients with uveitis, glaucoma, structural iris abnormalities, and low endothelial cell count.[7] Furthermore, since these lenses are not foldable, they require a larger wound size which increases the risk of intra-operative iris damage, uveal effusion, and spontaneous suprachoroidal haemorrhage -potentially severe complications that are already more frequent in these eyes -, and may also induce significant post-operative astigmatism unless a scleral tunnel approach is used.
Other options for the sulcus IOL could have been considered in this case, such as a simple acrylic 3-piece IOL. Although these IOLs were not developed for supplementary use in pseudophakic patients, there have been reports of their use in this manner with considerable safety and efficacy.[12,13] However, since the main surgeon had no experience in using these IOLs as supplementary IOLs it was decided to use an IOL that was specifically made for this purpose (Sulcoflex®). At last, there is also the option of addressing the high residual hyperopia with glasses or contact lens.
Even in cases where significant residual hyperopia remains, cataract surgery leads to a reduction in the thickness of glasses needed to correct any residual errors and improves the quality of life for patients. In our case, despite the implantation of two IOLs with the highest available power (+45 D in the bag and +10 D in the sulcus), significant residual hyperopia persisted. Despite this, the patient was satisfied with the refractive outcome and did not want to pursue further refractive surgery for the time being. Replacement of the supplementary sulcus IOL with a higher powered 3-piece sulcus IOL or iris-claw lens is certainly a possibility in this case, should the patient want to pursue further interventions. In conclusion, clear lens or cataract surgery in PM is particularly challenging not only due to the higher incidence of complications but also due to the uncertainty about the best refractive strategy.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Epidemiology of anophthalmia and microphthalmia: Prevalence and patterns in Texas, 1999-2009. Am J Med Genet A. 2018;176:1810-8.
- [CrossRef] [PubMed] [Google Scholar]
- Recognizing posterior microphthalmos. Ophthalmology. 2006;113:718.
- [CrossRef] [PubMed] [Google Scholar]
- High-hyperopia database, part I: Clinical characterisation including morphometric (biometric) differentiation of posterior microphthalmos from nanophthalmos. Eye (Lond). 2016;30:120-6.
- [CrossRef] [PubMed] [Google Scholar]
- Biometric and molecular characterization of clinically diagnosed posterior microphthalmos. Am J Ophthalmol. 2013;155:361-72.e7.
- [CrossRef] [PubMed] [Google Scholar]
- Cataract surgery in short eyes, including nanophthalmos: Visual outcomes, complications and refractive results. Clin Ophthalmol. 2021;15:4543-51.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of the refractive prediction determined by multiple currently available intraocular lens power calculation formulas in small eyes. Am J Ophthalmol. 2015;159:577-83.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison between refractive outcome of primary piggyback intraocular lens versus secondary lens iris claw lens in posterior microphthalmos. J Ophthalmol. 2019;2019:1356982.
- [CrossRef] [PubMed] [Google Scholar]
- Primary piggyback implantation of 3 intraocular lenses in nanophthalmos. J Cataract Refract Surg. 2007;33:727-30.
- [CrossRef] [PubMed] [Google Scholar]
- Refractive lens exchange and piggyback intraocular lens implantation in nanophthalmos: Visual and structural outcomes. J Cataract Refract Surg. 2017;43:1190-6.
- [CrossRef] [PubMed] [Google Scholar]
- The pathogenesis and treatment of complications in nanophthalmos. J Ophthalmol. 2020;2020:6578750.
- [CrossRef] [PubMed] [Google Scholar]
- Phacoemulsification with double-in-bag intraocular lens implantation in nanophthalmic eyes with angle-closure glaucoma. Int Ophthalmol. 2022;42:1861-6.
- [CrossRef] [PubMed] [Google Scholar]
- Refractive results: Safety and efficacy of secondary piggyback sensar™ AR40 intraocular lens implantation to correct pseudophakic refractive error. J Ophthalmol. 2016;2016:4505812.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical outcomes of piggybacking a one-piece IOL with a three-piece IOL in eyes with nanophthalmos. J Refract Surg. 2022;38:812-8.
- [CrossRef] [PubMed] [Google Scholar]






