Translate this page into:
A comparison of visual acuities obtained following biometry measurements by contact and immersion A-scan techniques
*Corresponding author: Hrishikesh Dilip Naik, Department of Ophthalmology, Byramjee Jeejeebhoy Govt. Medical College and Sassoon General Hospitals, Pune, Maharashtra, India. hrishikeshdnaik@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Darade DM, Naik HD, Puranik MA. A comparison of visual acuities obtained following biometry measurements by contact and immersion A-scan techniques. Glob J Cataract Surg Res Ophthalmol. 2024;3:10-5. doi: 10.25259/GJCSRO_28_2023
Abstract
Objectives:
This study aimed to obtain and compare ocular biometry measurements in terms of axial length, anterior chamber depth, and lens thickness by contact and immersion ultrasound techniques of A-scan and compare postoperative results in terms of visual acuity and spherical refractive error obtained by each of these techniques.
Materials and Methods:
This study was a prospective cohort and a comparative study that was done on 188 eyes under evaluation for cataract surgery. Out of these, 94 eyes were evaluated for biometry measurements using the immersion technique, and the rest were evaluated using the contact technique. Intraocular lens (IOL) powers were calculated using each of the above biometry parameters, and postoperative visual outcomes were obtained in terms of visual acuity and spherical refractive error. Results of demographic, clinical, and biochemical characteristics were expressed as range, mean, and median. For qualitative data, the Chi-square test and for comparison, Students’ unpaired t-tests were used. TAQhe statistical software SPSS Statistics was used to analyze the data. Microsoft Office Word and Excel 2019 were used to generate graphs and tables. P<0.05 was considered as significant.
Results:
Visual acuity was significantly better in the patients who underwent IOL implantation whose power was calculated by the immersion method on the 1st postoperative day. However, there was no significant difference in the final corrected visual acuity. Spherical refractive error at the end of 1 month was significantly greater in patients who underwent IOL power calculation by the contact method as compared to the immersion method.
Conclusion:
The immersion method of A-scan gives a lesser refractive error postoperatively and is a more precise method of biometry, though there is no difference in the final visual acuity obtained by either of these methods.
Keywords
Biometry
Eye axial length
Intraocular lenses
Cataract
Visual acuity
INTRODUCTION
Cataract is the major cause of blindness in the world and the most prevalent ocular disease. In India, cataract is the cause of bilateral blindness in 50–80% of patients.[1]
Modern-day cataract surgery is considered as a form of refractive surgery, aimed not only at restoring visual acuity but also at providing optimum vision by means of full refractive correction, which is achieved by implantation of a suitable intraocular lens (IOL) of a suitable power, which, in turn, is made possible as a result of the development of modern, accurate diagnostic and surgical techniques.
The principal steps in ocular biometry required to obtain the optimal power of the IOL and subsequently attain the desired postoperative refractive outcome involve the standardisation of techniques that ensure accurate measurements, which, in turn, provide a correct calculation of required IOL power for cataract surgery.[2,3] To predict the postoperative refraction with a fair degree of accuracy, several formulae have been developed to calculate the IOL power, and they require ocular biometry measurements in terms of axial length (AXL), average corneal curvature, anterior chamber depth, and lens thickness.[4]
AXL is the prime factor in IOL calculation. A 1 mm error in AL measurement results in a refractive error of approximately 2.35 d error of IOL power in an average eye of 23.5 mm and much more in short eyes.[5] Studies based on pre-operative and postoperative ultrasound biometry show that 54% of errors in predicted refraction after IOL implantation can be attributed to AXL measurement errors, 8% to corneal power measurement errors, and 38% to incorrect estimation of postoperative anterior chamber depth.[6] Furthermore, several studies have concluded that a whopping 87% of patients could achieve an outcome within ±1 dioptre (D) of the target with appropriate formula selection, proper AXL measurement, and optimised IOL constants.[7]
Ocular biometry values can be obtained either by contact (applanation), immersion, or optical methods.[8]
The contact/applanation technique is a widely used method that requires placing an ultrasound probe on the central cornea, whereas the immersion A-scan biometry utilises a saline-filled scleral (Prager) shell between the probe and the eye. The optical method is a non-contact technique that utilises light by partial coherence interferometry and measures the AXL along the visual axis.[9]
The gold standard technique, due to the least operator dependency, is the optical method by partial coherence laser interferometry. Unfortunately, it is limited in not being widely available nor economical on account of its higher cost, and the limit of its applicability in the usually dense cataract makes the ultrasonography method more appropriate.
A-scan ultrasonography involves passing an ultrasound beam generated by a piezoelectric crystal and delivered through a transducer through the eye, and as the beam returns to the probe after hitting intraocular structures, a set of ocular spikes is displayed on the instrument screen representing the cornea to the orbital fat.[10] These spikes represent the differential return of the ultrasonic waves by the varying tissue types.
The immersion method of the A-scan is supposed to have greater accuracy than the contact method due to the absence of corneal compression-induced error in the AXL in the former.[11]
This study aims to compare the postoperative visual outcome evaluated in terms of visual acuity and spherical refractive error using biometry measurements obtained by contact and immersion ultrasound techniques of the A-scan.
MATERIALS AND METHODS
The present study was conducted at a tertiary care hospital during the period from June 2018 to November 2020. A synopsis of the study protocol was submitted to the Institutional Ethics Committee, and approval was obtained. This was a 2-year prospective, comparative, observational, and cross-sectional study.
Patients visiting the department of ophthalmology and admitted to the ward of a tertiary care hospital who satisfied the inclusion criteria were enrolled in the study. Ninety-four patients for each group for a total of 188 patients, were selected by convenience sampling for comparison based on the following eligibility criteria.
Subjects were eligible if the following criteria were met
Patients with an immature senile cataract
Patients above the age of 15.
Subjects who met any of the following criteria were excluded from this study
Patients with a mature senile cataract
Patients with abnormal findings on fundoscopy
Patients under the age of 15
Complicated postoperative cases
Patients with a subluxated or dislocated cataract
Patients with any other anterior and/or posterior segment pathologies.
This study was a prospective, comparative, observational, and cross-sectional study done on 188 eyes under evaluation for cataract surgery at a tertiary care hospital. The patient profile included their age, sex, and AXL. A detailed ophthalmic examination was done. Out of 188 patients, 94 eyes were evaluated for biometry measurements using the immersion technique, and the rest 94 by the contact technique. IOL powers were calculated using each of the above biometry parameters, and postoperative visual outcomes were obtained in terms of visual acuity and spherical refractive error on postoperative day one and after a follow-up of 1 month. Biometry measurements were made on Echorule Pro Biometer (BioMedix Optotechnik and Devices Pvt. Ltd, Bengaluru, India). The biometry measurements were performed by the same individual.
A detailed history was elicited from all the patients or their relatives and/or from medical records. A meticulous systemic examination was conducted on all the patients. Fasting and post-prandial blood sugar levels were checked. Each patient was then subjected to a comprehensive general physical examination followed by a detailed ocular examination.
Detailed preliminary history
Significant systemic and family history
Examination: General/Systemic/Ocular.
Detailed ocular examination included
Visual acuity testing (uncorrected and best-corrected) using the Snellen chart
Colour vision
Intraocular pressure measurement using non-contact tonometry/applanation tonometry 4. Extraocular motility assessment
Slit lamp biomicroscopy
Dilated fundus examination using direct and/or indirect ophthalmoscopy. It also included slit-lamp biomicroscopy using a 90-D lens.
Optical coherence tomography
Automated refractometry (AR)/retinoscopy
A-Scan biometry.
RESULTS
In the contact A-scan group, 23 patients were in the age group 51–60 years (24.5%), and 45 patients were in the age group 61–70 years (47.9%). Twenty patients (21.24 %) were in the age group of 71–80 years of age. Four patients were in the age group 41–50 years, whereas only two patients were more than 80 years old. Similarly, in the immersion group, 19 patients (20.4%) were in the age group of 51–60 years, 43 patients (45.6%) were in the age group of 61–70 years, and 25 patients (26.6%) were in the age group of 71–80 years, whereas only one patient was above the age of eighty, and six patients (6.4%) were <50 years of age [Chart 1]. In both group, male patients were more than females [Chart 2].
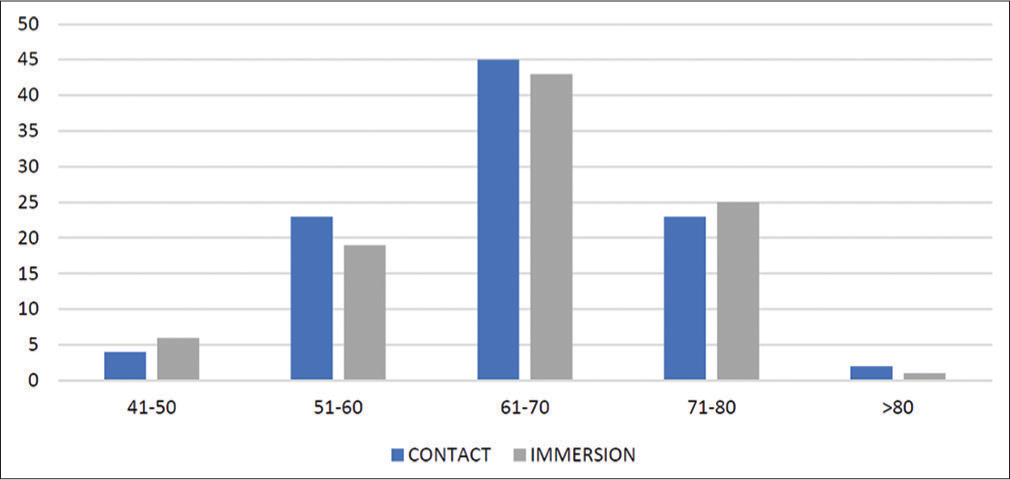
- Distribution of study participants according to age.
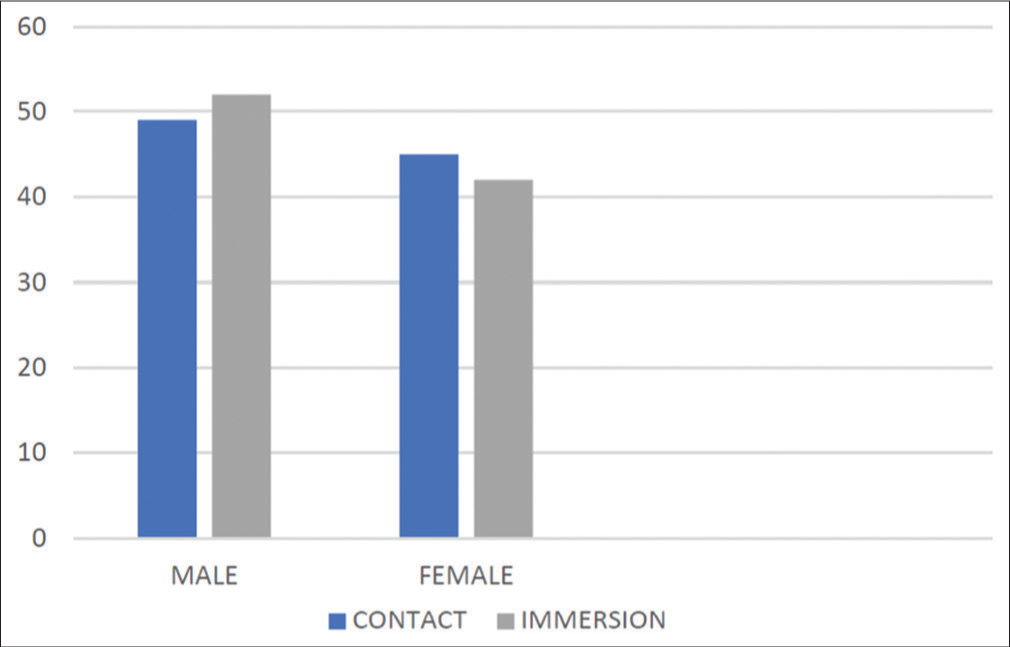
- Distribution of study participants according to gender.
In the contact group, 55 (58.5%) patients had the right eye, and 39 (41.5%) had the left eye involved and operated, whereas in the immersion group, 67 patients (71.3%) had cataract in the right eye which was operated and 27 (28.7%) were operated in the left eye. The differences between the two groups were not statistically significant (P > 0.05) [Chart 3].
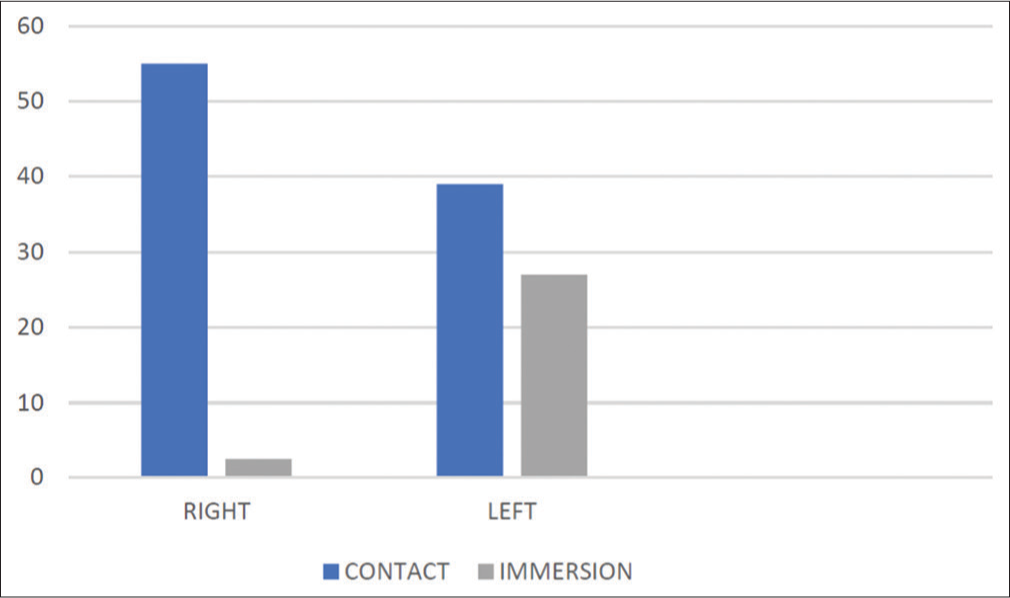
- Distribution of study participants according to side operated.
In the contact group, 3 (3.2%) patients had posterior subcapsular cataract and 69 (73.4%) patients were having both. Twenty-two (23.4 %) patients were having nuclear sclerosis. Similarly, in immersion group, 5 (5.3%) patients were having posterior subcapsular cataract, followed by 74 (78.8%) patients having both types. Fifteen (15.9%) patients were having nuclear sclerosis. There were no patients in both the groups having isolated peripheral cortical cataract. The difference between the two groups was not statistically significant (P > 0.05) [Chart 4].
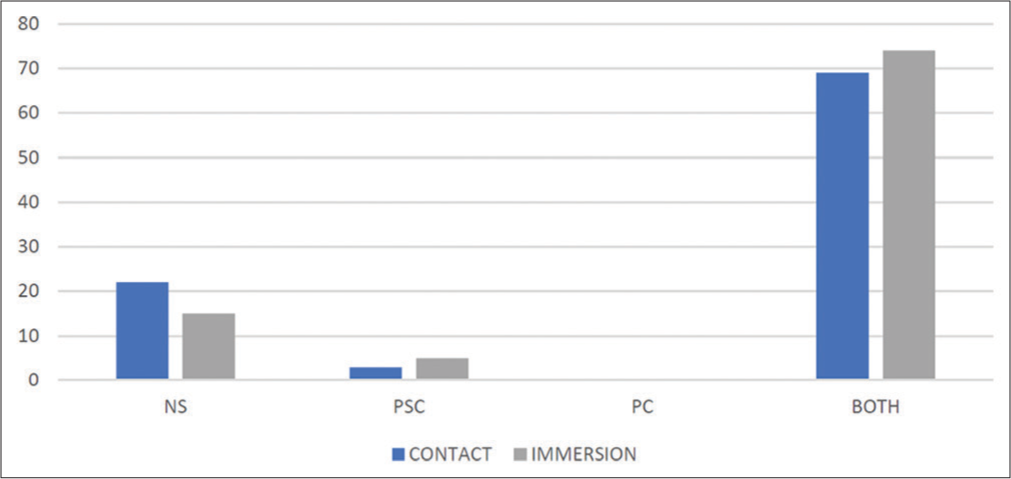
- Distribution of study participants according to type of cataract. NS: Nuclear sclerosis, PSC: Posterior subcapsular cataract, PC: Peripheral cortical.
Sixty-two (66%) of the contact group underwent small-incision cataract surgery (SICS) and 32 (34%) underwent clear corneal phacoemulsification (CCP). Similarly, 58 patients corresponding to 61.7% of cases in the immersion group underwent SICS and 36 patients (38.3%) underwent CCP. The differences between the two groups were not statistically significant (P > 0.05) [Chart 5].
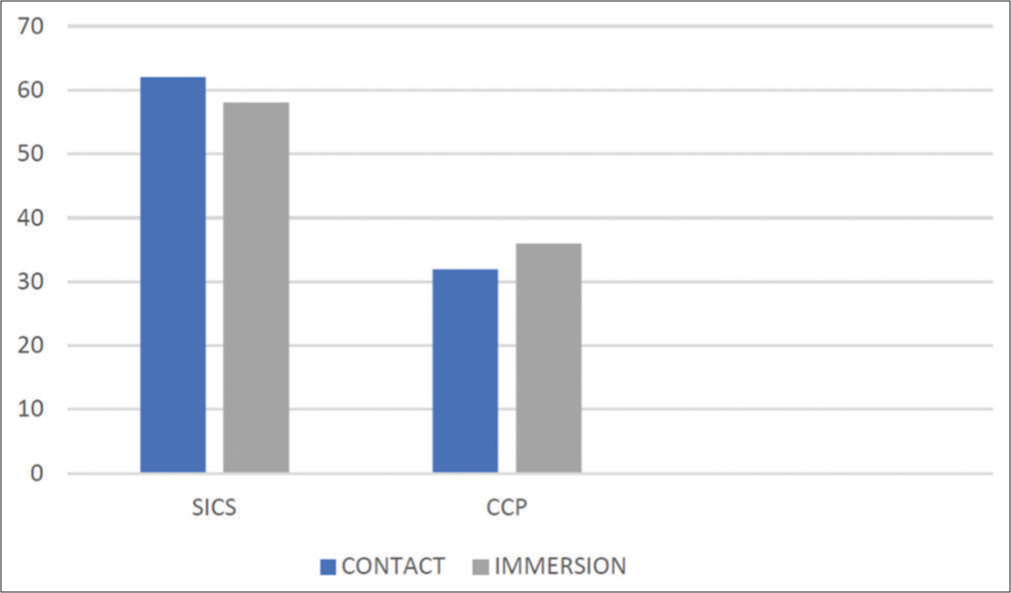
- Distribution of study participants according to surgical procedure done, SICS: Small incision cataract surgery, CCP: Clear corneal phacoemulsification.
The mean AXLs in the contact and immersion groups were 23.21 ± 0.76 mm and 23.40 ± 0.86 mm, respectively. Similarly, the mean IOL power in the contact group was 22.47 ± 2.13 D, and in the immersion group was 22.37 ± 2.39 D. This difference was not statistically significant [Chart 6].
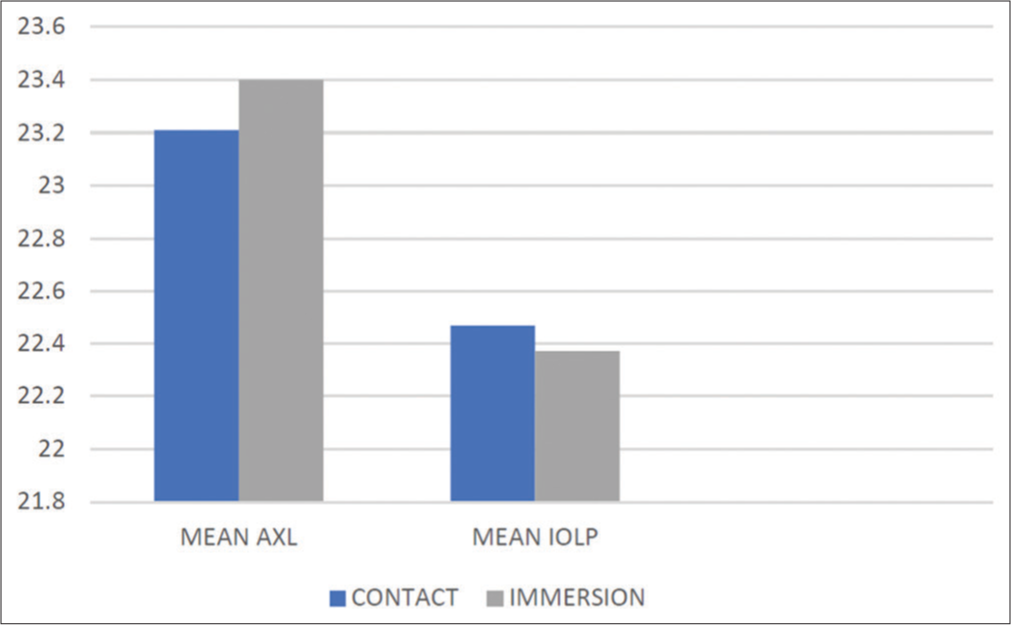
- Comparison of mean axial length (AXL) and intraocular lens power (IOLP) between the two groups.
On the 1st postoperative day, the difference between both the uncorrected and best-corrected visual acuities in both groups was statistically significant (P < 0.05). At one month, the difference between uncorrected visual acuities in both groups was also significant. However, there was no statistically significant difference in the best-corrected visual acuity between both groups at one month [Chart 7 and Table 1].
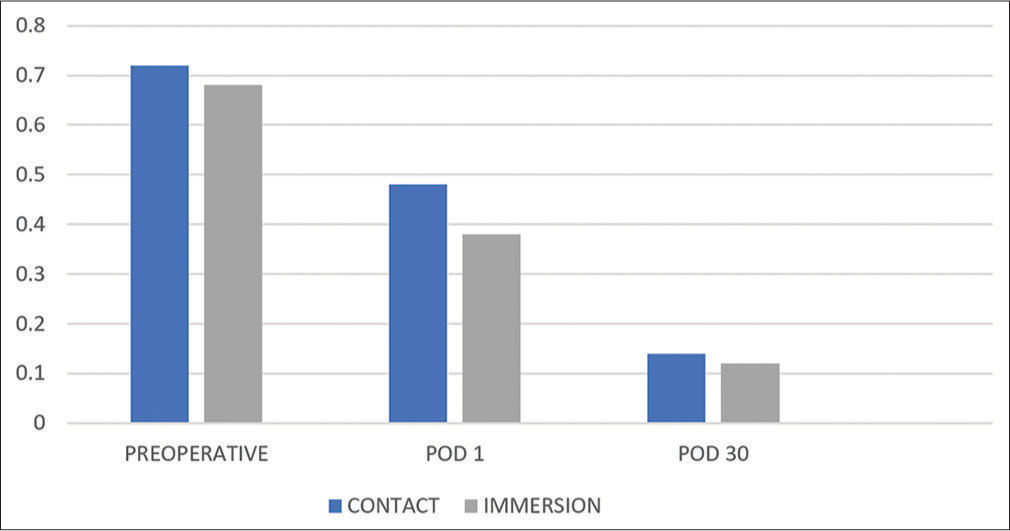
- A comparison of pre-operative and postoperative visual acuities between both groups, POD: Post operative day.
| Contact | Immersion | P-value | |||
|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | ||
| Pre-op | |||||
| UCVA (LogMAR) | 0.89 | 0.29 | 0.82 | 0.3 | 0.1 |
| BCVA (LogMAR) | 0.72 | 0.27 | 0.68 | 0.29 | 0.3 |
| POD 1 | |||||
| UCVA (LogMAR) | 0.58 | 0.14 | 0.44 | 0.18 | <0.001 |
| BCVA (LogMAR) | 0.48 | 0.13 | 0.38 | 0.18 | <0.001 |
| POD 30 | |||||
| UCVA (LogMAR) | 0.28 | 0.09 | 0.19 | 0.14 | <0.001 |
| BCVA (LogMAR) | 0.14 | 0.09 | 0.12 | 0.11 | 0.17 |
| Spherical refractive error (absolute) | 1.29 | 0.26 | 0.48 | 0.27 | <0.001 |
| Median | 1.25 | 0.5 | <0.001 | ||
UCVA: Uncorrected visual acuity, BCVA: Best corrected visual acuity, POD: Postoperative day, SD: Standard deviation, LogMAR: Logarithm of the Minimum angle of resolution.
Spherical refractive error was significantly lower in the immersion group as compared to the contact group after one month postoperatively (P < 0.05) [Chart 8].
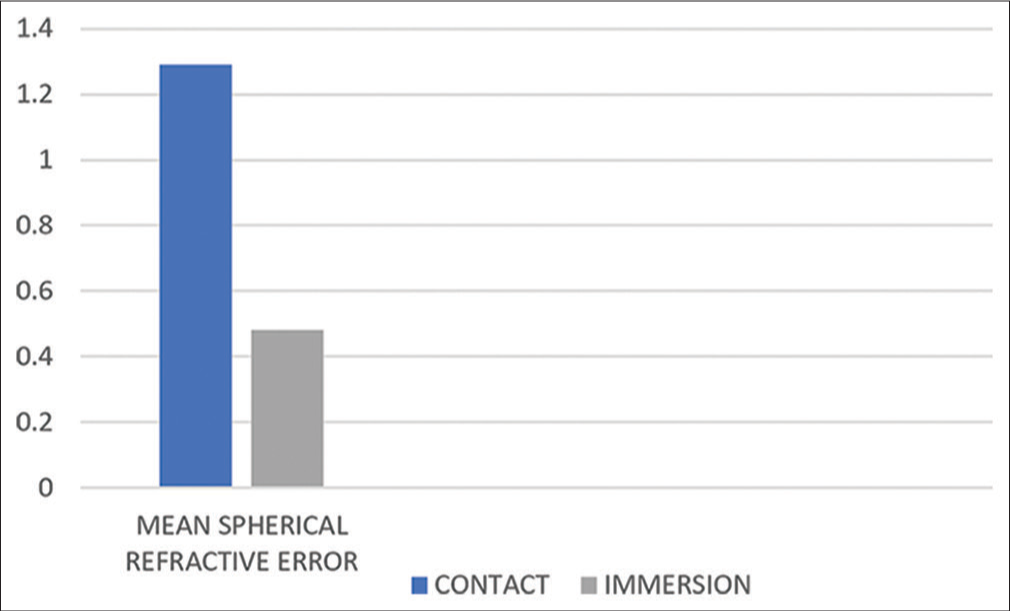
- Comparison of spherical refractive error between both groups at postoperative day 30.
DISCUSSION
In India, senile cataract is responsible for more than half of cases of visual impairment.[1] In the present study, ocular biometry measurements in terms of AXL, anterior chamber depth, and lens thickness were measured using the contact and immersion techniques of A-scan ultrasonography. Furthermore, postoperative results in terms of visual acuity and spherical refractive error have been compared with measurements obtained by each of these techniques. Results obtained in the present study have been compared and contrasted with previous studies regarding the same.
In the present study, the age group of 61–70 years was the most affected, with mean ages of 65.37 and 65.76, respectively. There were more male patients than female patients. In related studies, a similar pattern of age distribution has been found.[12,13]
Mixed-grade cataract was the most common, followed by isolated nuclear sclerosis and posterior subcapsular cataract.
It was found that the immersion method gave a lesser spherical refractive error compared to the contact method. Furthermore, the immersion method had better repeatability, as evidenced by lesser deviation from the mean. These study results were comparable with similar studies.[12,13] Furthermore, there was no significant difference in the final visual acuity obtained by either of these methods, which is also correlated with similar studies.[14] However, this was only applicable to adult eyes, as studies by Ben-Zion et al.[15] found no difference in AXL and postoperative refractive error in paediatric eyes too by either method.
The immersion method gave a shorter AXL than the contact method by an average of 0.19 mm. Comparable results have been obtained by Schelenz and Kammann[16] and Olsen and Nielsen in their study.[17] There was no significant difference in the corneal curvatures between the two groups, also correlated by a related study.[18]
Age at surgery, side operated, gender, grade of immature cataract, and type of surgery (SICS or CCP) did not influence the ultimate spherical refractive error obtained or the final best-corrected visual acuity, as evidenced by Murphy et al. in their research.[19]
Immersion biometry even gives comparative results with the precise final postoperative refractive outcome at a lesser cost compared to the industry standard optical biometry,[18] even with fourth-generation IOL calculation formulae.[20] This might be especially useful in dense ocular media where optical biometry is not possible. It has even been experimentally proved that immersion biometry was less affected by lateral movement and tilt of the probe.[10] Newer IOL calculation formulae also need to be adapted to the immersion technique due to its accuracy.[21]
CONCLUSION
Based on analyses with multiple research projects, the immersion method is a more precise and accurate method of A-scan biometry with better repeatability and operator dependence than the contact method. The present study is limited by a relatively narrow range of AL in adult eyes with a limited sample size. Therefore, it is difficult to apply the results of our study to extremely short, long, or paediatric eyes. Furthermore, as we included only uncomplicated cataract surgeries, no predictions can be made for eyes with unusual keratometry or skewed anterior-to-posterior segment ratio.
Manual SICS has comparable results to phacoemulsification concerning the visual outcome, safety, complication rate, and even surgical-induced astigmatism, with less dependence on expensive equipment and supplies, at times outperforming phacoemulsification in both efficacy and safety.
Acknowledgment
The authors would like to thank Dr. SV Ambekar, Professor and Head of Department of Ophthalmology.
Ethical approval
The author(s) declare that they have taken the ethical approval from IEC. No. BJGMC/IEC/Pharmac/D -1118203-203 dated 27/11/2018.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Current estimates of blindness in India. Br J Ophthalmol. 2005;89:257-60.
- [CrossRef] [PubMed] [Google Scholar]
- Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008;34:368-76.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of contact and immersion techniques of ultrasound biometry in terms of target postoperative refraction. Cesk Slov Oftalmol. 2009;65:143-6.
- [Google Scholar]
- Intraocular lens calculation and ultrasound biometry: Immersion and contact procedures. Klin Monatsbi Augenheilkd. 1998;213:161-5.
- [CrossRef] [PubMed] [Google Scholar]
- Basic and clinical science course, section 3. American Academy of Ophthalmology. :211-23.
- [Google Scholar]
- IOL master optical biometry vs. Conventional ultrasound in intraocular lens calculations in high myopic eyes. AIOC. 2009;4:136-9.
- [Google Scholar]
- Benchmark standards for refractive outcomes after NHS cataract surgery. Eye (Lond). 2007;23:149-52.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of operative experience on the performance of ultrasound biometry compared to optical biometry before cataract surgery. J Cataract Refract Surg. 2003;29:1950-5.
- [CrossRef] [PubMed] [Google Scholar]
- Immersion A-scan compared with partial coherence interferometry. J Cataract Refract Surg. 2002;28:239-42.
- [CrossRef] [PubMed] [Google Scholar]
- The contact and immersion ultrasound methods compared using the ray tracing method. Opt Appl. 2010;40:77-92.
- [Google Scholar]
- Comparison of axial length and anterior chamber depth measurement among optical, applanation and immersion biometry. J Shaheed Suhrawardy Med Coll. 2019;11:69-4.
- [CrossRef] [Google Scholar]
- Comparison of ocular biometry measurements by applanation and immersion A-scan techniques. J Curr Ophthalmol. 2015;27:110-4.
- [CrossRef] [PubMed] [Google Scholar]
- Immersion vs contact biometery for axial length measurement before phacoemulsification with foldable IOL. Pak J Ophthalmol. 2009;25:4.
- [Google Scholar]
- Accuracy of IOL calculations in children: A comparison of immersion versus contact A-scan biometery. J AAPOS. 2008;12:440-4.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of contact and immersion techniques for axial length measurement and implant power calculation. J Cataract Refract Surg. 1989;15:425-8.
- [CrossRef] [PubMed] [Google Scholar]
- Immersion versus contact technique in the measurement of axial length by ultrasound. Acta Ophthalmol. 1989;67:101-2.
- [CrossRef] [PubMed] [Google Scholar]
- Refractive outcome after phacoemulsification using optical biometry versus immersion ultrasound biometry. Egypt J Hosp Med. 2019;75:2806-12.
- [CrossRef] [Google Scholar]
- Refractive error and visual outcome after cataract extraction. J Cataract Refract Surg. 2002;28:62-6.
- [CrossRef] [PubMed] [Google Scholar]
- Immersion biometry for intraocular lens power calculation with fourth-generation formulas. Clin Ophthalmol. 2020;14:2159-62.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound biometry and intraocular lens power calculation: Comparative study of the contact and immersion techniques. Rev Bras Oftalmol. 2009;68:212-5.
- [CrossRef] [Google Scholar]







