Translate this page into:
Visual acuity and vision-related quality of life outcomes following cataract surgery in Ebola virus disease survivors
*Corresponding author: Steven Yeh, Department of Ophthalmology and Visual Sciences, Truhlsen Eye Institute, University of Nebraska Medical Center, Omaha, United States. syeh@unmc.edu
-
Received: ,
Accepted: ,
How to cite this article: Taraborelli D, Thomas JJ, Kim L, Fashina T, Hayek B, Mattia JG, et al. Visual acuity and vision-related quality of life outcomes following cataract surgery in Ebola virus disease survivors. Glob J Cataract Surg Res Ophthalmol 2023;2:23-9.
Abstract
Objectives:
The objectives of this study were to assess relationships between vision-related quality of life (QoL) and visual acuity (VA) in Ebola virus disease (EVD) survivors after cataract surgery in the Ebola Viral Persistence in Ocular Tissues and Fluids (EVICT) Study.
Materials and Methods:
EVD survivors with undetectable Ebola virus (EBOV) ribonucleic acid in their aqueous humour were eligible to receive manual small-incision cataract surgery (MSICS). Among those that received surgery, assessments of VA and vision-related QoL were assessed pre-and post-cataract surgery. VA was converted from units on a tumbling ‘E’ chart to the logarithm of the minimal angle of resolution VA (logMAR VA). Vision-related QoL was assessed using the 25-item National Eye Institute Visual Function Questionnaire (NEI VFQ-25). Linear regression was used to evaluate the associations between VA and vision-related QoL. P = 0.05 was considered statistically significant for all analyses.
Results:
Thirty-four EVD survivors underwent cataract surgery in the EVICT study. Before MSICS, the mean logMAR VA was 2.24 (standard deviation [SD]: 0.98), and the mean NEI-VFQ-25 composite score was 54 (SD: 15); however, there was no significant association between the pre-surgery measurements (average difference in VA/10 unit increase in NEI-VFQ-25: −0.04, 95% confidence interval (CI): −0.33–0.26, P = 0.80). There was a significant improvement in logMAR VA after MSICS (mean: 1.6, P < 0.001), but there was no significant change in the NEI-VFQ-25 composite (−0.87, 95% (CI): −10.32–8.59, P = 0.85). None of the subscales showed significant improvements (P > 0.12 for all); however, the magnitude of the mean change for distance activities (6.65), near activities (6.76), general vision (−7.69), social functioning (−9.13) and colour vision (13.33) met the criteria for a clinically meaningful difference (4–6). In the subset with paired measurements (n = 16), there were no significant association changes in logMAR VA and NEI VFQ-25 composite scores (P > 0.12 for all).
Conclusion:
Following cataract surgery, VA in EVD survivors improved, but these improvements were not reflected in NEI VFQ-25 composite scores or specific subscales; however, the small sample size limits generalizability absent more research. Differences in sociocultural context and activities that affect the QoL in resource-limited areas may contribute to the limitations seen with NEI VFQ-25. In addition, better eye dominance could contribute to any lack of association as NEI VFQ-25 evaluates vision as a whole. Further, assessment of factors contributing to improved QoL may help to define the impact of vision health in varied environments.
Keywords
Ebola virus disease (EVD)
Uveitis
Cataract surgery
Visual function questionnaire
Vision-related quality of life
INTRODUCTION
The most significant Ebola virus disease (EVD) outbreak in history, which resulted in over 28,000 cases from 2013 to 2016 in Western Africa,[1] left a large cohort of survivors requiring ongoing convalescent care for a variety of newly characterised post-acute clinical sequelae, including ocular complications.[2] During and after the outbreak, >15,000 survivors were estimated to be at risk for post-EVD sequelae.[3] Unfortunately, the number of EVD survivors at risk continues to increase: six additional EVD outbreaks have been declared since 2016, including the second largest outbreak in history in the Democratic Republic of the Congo (DRC) in 2018–20, two independent outbreaks in DRC in 2021, in Guinea in 2021 and two more independent outbreaks in DRC in 2022.[4] Finally, an ongoing outbreak of Sudan virus disease in Uganda raises questions of similar risk in these survivors of a closely related filovirus disease.[4]
Uveitis is the most common ocular complication for EVD survivors, with approximately 13–34% developing the disease.[5,6] Uveitis is a potentially blinding inflammatory disease and accounts for as much as 25% of blindness in the developing world.[7] Vision loss attributable to complications of uveitis includes cataracts, chorioretinal scarring, retinal detachment and glaucoma.[8] Within a cohort of EVD survivors evaluated in Liberia, a visual acuity (VA) of 20/400 or worse was observed at initial presentation in nearly 40% of EVD survivors with signs of uveitis, highlighting the devastating effects post-EVD uveitis can have on vision, particularly in areas with limited resources and health care access.[5] Since uveitis is one of the most common post-Ebola sequelae, and if left untreated may lead to cataracts, it is important to understand the burden of vision loss on the ability to work, routine activities of daily living and quality of life (QoL) in this population.
The Ebola Virus Persistence in Ocular Tissues and Fluids (EVICT) study[9] conducted between 2016 and 2017 in Sierra Leone was the first study to evaluate the safety, feasibility and potential for cataract surgery to restore vision in EVD survivors.[8] Because EBOV has been documented to persist in the aqueous humour during EVD convalescence,[10] cataract surgery in the EVICT study involved a two-stage process in preventing the risk of contact with undetected virus, including (1) assessment of aqueous humour for EBOV ribonucleic acid (RNA) by real-time reverse transcriptase-polymerase chain reaction testing (RT-PCR) (2) manual small-incision cataract surgery (MSICS) if EBOV RNA was not detected in the aqueous humour. The EVICT and PREVAIL VII studies[11] have described visual improvement following cataract surgery in EVD survivors. However, no known studies have evaluated the changes in this population’s vision-related QoL metrics. In this study, we assessed the impact of cataract surgery on vision-related QoL for EVD survivors in the EVICT study. We also analysed the relationship between VA change and vision-related QoL following cataract surgery.
MATERIALS AND METHODS
Study design
Details of the design of the EVICT study have been previously described.[9] In brief, participants were identified for the EVICT study through the Sierra Leone Association of Ebola Survivors and the Ministry of Health (MOH) National Eye Program Database between June 2016 and August 2017. EVICT included EVD survivors of all ages with either EBOV-Immunoglobulin G seropositivity or an Ebola Treatment Unit (ETU) discharge certificate documenting prior virologic confirmation of EVD and eye disease that required intraocular surgical intervention. Written and witnessed informed consent for the study was conducted by the physician performing the procedures with medical interpretation in Krio, English, or the patient’s dialect. The Institutional Review Board approval was obtained from Emory University and the Office of Ethics and Scientific Review Committee, Sierra Leone MOH and Sanitation. Human research was conducted according to the Tenets of the Declaration of Helsinki, and informed consent was obtained with the assistance of Sierra Leonean interpreters in the native dialect of enrolled patients.
This analysis focuses on enrolled EVICT participants who qualified for cataract surgery and in whom EBOV RNA was not detected in ocular fluid [Figure 1]. RT-PCR was done as it has been shown that virus can exist undetected in fluids such as semen, breast milk and the aqueous humour of the eye.[1] Participants who opted for surgery received MSICS with intraocular lens (IOL) implantation. Cataract was classified as nuclear sclerotic, posterior subcapsular, anterior subcapsular, uveitic white cataract, and uveitic white cataract with anterior capsular fibrosis and graded from one to four. The RT-PCR and MSICS protocol has been previously reported in the Supplemental Appendix of the EVICT Study: RT-PCR and Cataract Surgery Outcomes in Ebola Survivors in Sierra Leone.[9] Briefly, patients undergoing MSICS were anesthetised with a retrobulbar block with 2% lidocaine without epinephrine. Using aseptic surgical technique with an eyelid speculum, 100–150 microliters of aqueous humour was aspirated for RT-PCR analysis. Viscoelastic was instilled into the anterior chamber. Conjunctival peritomy was fashioned at the superior corneoscleral limbus. Following low-temperature cautery to maintain homeostasis, a frown shaped incision was made and a microkeratome was used to enter the anterior chamber. A cystotome was used to fashion ‘envelope’ shaped capsulorhexis, after which the cystotome was used to engage and remove the lens nucleus. The cortical remnants were removed with manual aspiration and irrigation with balanced saline solution. An IOL of predetermined lens power to achieve a post-operative refractive error of −0.50– 1.00 Diopters was inserted. A 10-0 nylon suture was placed if needed and subconjunctival ceftazidime and dexamethasone injections were instilled. An eye patch and protective shield were subsequently secured for removal the following day. Post-operative VA evaluations were conducted at 1 day, week, month and 3–4 months. Post-surgery National Eye Institute 25-Item Visual Function Questionnaire (NEI VFQ-25) assessments were conducted at 1 month and 3–4 months.
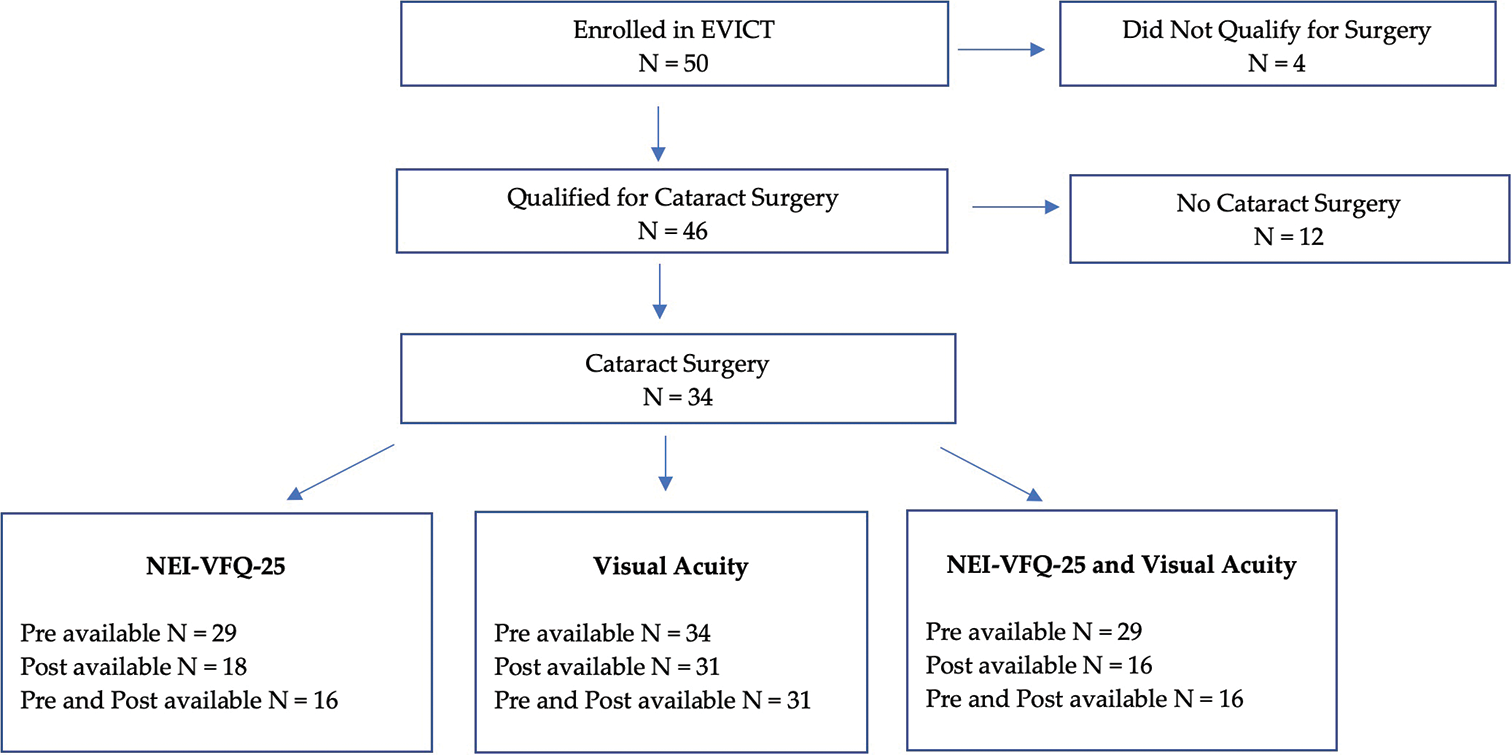
- CONSORT diagram – Ebola Viral Persistence in Ocular Tissues and Fluids (EVICT) participants included in analysis of visual acuity and national eye institute visual function questionnaire 25 vision-related quality of life before and after cataract surgery. N: number of subjects, NEI-VFQ25: National Eye Institute Visual Functional Questionnaire 25.
Data collection and outcomes
At enrolment, data were collected on age, sex and medical history. VA and vision-related QoL were assessed preand post-surgical intervention. VA was assessed using the Tumbling ‘E’ chart and converted to the logarithm of the minimal angle of resolution (logMAR) for statistical analyses of the study eye. Counting fingers and hand motions were converted to logMAR as previously described.[12] The eye with worse vision was selected as the study eye. Vision-related QoL was assessed using the NEI VFQ-25.[12,13] A trained staff member with oversight from physician investigators administered the questionnaire to subjects in the EVICT study in English, Krio, or the patient’s dialect using interpretation. The NEI VFQ-25 consists of a base set of 25 vision-targeted questions representing 11 vision-related constructs, including a single-item general health rating question that does not contribute to the calculation of the overall composite score. The NEI VFQ-25 was scored by methods previously described by Mangione et al.[13,14] The original numeric response score from the questionnaire was recoded to a value of 0–100 depending on the item numbers per category and averaged based on the algorithm to generate the NEI VFQ-25 sub-scale scores. The overall composite score was determined by averaging the vision-related sub-scale scores and excluding the general health score. The general health score was determined by converting the original numeric response value of 1–5 to its corresponding recoded score of 0–100. Higher scores represent a better vision-related QoL. Per the NEI VFQ-25 scoring algorithm guidelines described by Mangione, questions that are left unanswered are not included when calculating the sub-scale scores.[13] The subscales that have at least one question answered can be used to generate a sub-scale score, with scores representing the average of all questions answered.
Statistical analysis
Baseline demographics and ocular characteristics, such as logMAR, VA time from EVD diagnosis to cataract surgery, NEI VFQ-25 and medical history, were summarised using means, standard deviations (SDs) and percentages for categorical variables. Paired t-tests were used to compare the pre-and post-cataract surgery measurements. Linear regression was performed to evaluate the association between NEI VFQ-25 scores and logMAR VA. Statistical analyses were performed using STATA 15.0. P < 0.05 was considered statistically significant for all analyses.
RESULTS
Of the 50 participants enrolled in EVICT,[9] 46 EVD survivors were eligible for cataract surgery, and 34 of those eligible proceeded with cataract surgery [Figure 1]. Surgeries were uncomplicated with no instances of posterior capsular rupture. Baseline demographic and medical characteristics for survivors who underwent cataract surgery were similar to those who chose not to participate [Table 1]. Of the 34 EVD survivors who underwent cataract surgery, the pre-surgery mean logMAR VA in the worst operated study eye was 2.24 (SD 0.98), and the pre-surgery mean NEI VFQ-25 general health and composite scores were 51 (SD: 25) and 54 (SD: 15), respectively [Table 2]. Participants scored highest on the subscales related to colour vision (Mean: 78, SD: 24) and social functioning (Mean: 76, SD: 18). Participants scored the lowest on subscales related to driving (Mean: 32, SD: 19) and mental health (Mean: 35, SD: 20). There was no significant association between pre-surgery logMAR VA and NEI VFQ-25 composite scores (average difference in VA per 10 units increase in NEIVFQ-25: −0.04, 95% CI: −0.33 to 0.26, [Figure 2]).
| Overall | Cataract surgery | No cataract surgery | P-value | |
|---|---|---|---|---|
| n=46 | n=34 | n=12 | ||
| Female, n(%) | 31 (67) | 20 (59) | 11 (92) | 0.41 |
| Age in years | ||||
| Mean±SD | 29±15 | 27±15 | 31±15 | 0.5 |
| Missing, n(%) | 7 (15) | 4 (12) | 2 (17) | |
| logMAR visual acuity | ||||
| Study Eye, Mean±SD | 2.24±0.97 | 2.24±0.98 | 2.4±0.99 | 0.95 |
| Fellow Eye, Mean±SD | 0.124±0.35 | 0.159±0.41 | 0.030±0.07 | 0.10 |
| Medical history, n(%) | ||||
| Typhoid | 1 (2) | 1 (3) | 0 | >0.99 |
| Malaria | 22 (48) | 14 (41) | 8 (67) | 0.18 |
| Asthma | 1 (2) | 1 (3) | 0 | >0.99 |
n: Number, %: Percent, SD: Standard deviation, EVICT: Ebola Viral Persistence in Ocular Tissues and Fluids. Missing data for the following variables: Study eye overall (0) study eye cataract surgery (0), study eye no cataract surgery (0), fellow eye overall (3, 6.5%), fellow eye cataract surgery (0), fellow eye no cataract surgery (1, 8.3%), logMAR: log of minimum angle of resolution
| Pre-cataract surgery | Post-cataract surgery | Post-cataract – pre-cataract surgery difference | P-value | ||||
|---|---|---|---|---|---|---|---|
| n | MeanSD | n | MeanSD | n | Mean (95% CI) | ||
| BCVA (logMAR units) | |||||||
| Study eye | 34 | 2.17±1.1 | 31 | 0.63±0.89 | 31 | 1.6 (1.22, 2.01) | <0.001 |
| NEI-VFQ-25 category | |||||||
| General health | 29 | 51±25 | 20 | 55±17 | 16 | 1.56 (−15.61, 18.74) | 0.85 |
| Composite | 29 | 54±15 | 19 | 57±17 | 15 | −0.87 (−10.32, 8.59) | 0.85 |
| General vision | 23 | 57±14 | 20 | 69±10 | 13 | −7.69 (−18.20, 2.82) | 0.14 |
| Ocular pain | 29 | 47±24 | 20 | 50±18 | 16 | −0.938 (−15.63, 13.76) | 0.89 |
| Near activities | 29 | 60±25 | 20 | 58±22 | 16 | 6.76 (−11.23, 24.74) | 0.44 |
| Distance activities | 29 | 60±24 | 20 | 63±22 | 16 | 6.65 (−7.31, 20.61) | 0.33 |
| Social functioning | 29 | 76±18 | 20 | 72±22 | 16 | −9.13 (−2.65, 20.90) | 0.12 |
| Mental health | 29 | 35±20 | 20 | 40±15 | 16 | −1.88 (−14.83, 11.08) | 0.76 |
| Role difficulties | 29 | 47±16 | 20 | 49±22 | 16 | −3.13 (−18.07, 11.82) | 0.66 |
| Dependency | 28 | 41±24) | 20 | 44±19 | 15 | −3.89 (−18.48, 10.69) | 0.58 |
| Driving | 6 | 32±19 | 5 | 78±27 | 2 | −29.35 (−190.08, 131.38) | 0.26 |
| Colour vision | 28 | 78±24 | 20 | 69±24 | 15 | 13.33 (−9.39, 36.06) | 0.23 |
| Peripheral vision | 27 | 56±27 | 20 | 64±19 | 14 | 0 (−19.61, 19.61) | >0.99 |
n: Number, SD: Standard deviation, NEI-VFQ: National eye institute visual function questionnaire, BCVA: best-corrected visual acuity, logMAR: Logarithm of the minimal angle of resolution

- Comparison of visual acuity and national eye institute visual function questionnaire 25 vision-related quality of life before cataract surgery. Note: The blue dotted line indicates the general trend of the data. logMAR: log of minimum angle of resolution, VFQ-25: National Eye Institute Visual Function Questionnaire 25.
Of the 34 EVD survivors who underwent cataract surgery, the majority had pre-and post-surgery logMAR VA (n = 31, 91%) data available, while fewer (n = 16, 47%) had pre-and post-surgery data for NEI VFQ-25 scores [Figure 1]. There was a significant improvement in logMAR VA following surgery in the operated eye (∆ mean: 1.6, SD: 1.09; 95% CI: 1.22, 2.01, P < 0.001). The NEI VFQ-25 general health, composite and sub-scale scores were comparable to pre-and post-cataract surgery [Table 2]. Within the general vision categories, numerical increases were observed in distance activity (mean change [∆]: 6.65), near VA (∆: 6.76), social functioning (∆: −9.13) and colour vision (∆: 13.3), although changes were not statistically significant. Among the 16 EVD survivors with paired measurements, the change in VA was not found to be associated with the change in NEI VFQ-25 composite scores (average change in VA per unit change in NEI VFQ-25: −0.02, 95% CI: −0.11–0.06, P = 0.54, [Figure 3]).
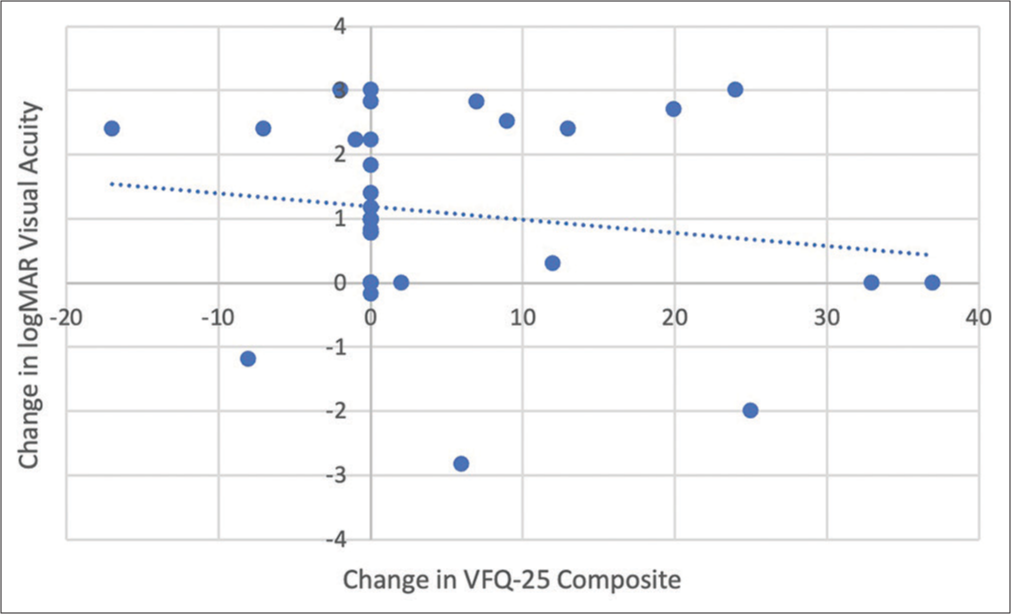
- Comparison of the changes in visual acuity and national eye institute visual function questionnaire vision-related quality of life after cataract surgery. Note: The blue dotted line indicates the general trend of the data. logMAR: log of minimum angle of resolution, VFQ-25: National Eye Institute Visual Function Questionnaire 25.
DISCUSSION
The association between VA and vision-related quality-of-life metrics as measured by the NEI VFQ-25 score has been demonstrated in prior studies of uveitis[15] in general and after cataract surgery.[16,17] The National Institutes of Health-funded PREVAIL III study similarly assessed uveitis and cataract in Liberian EVD survivors.[6] They also prospectively showed the pre-operative absence of EBOV RNA in aqueous humour and significant VA improvement in EVD survivors following cataract surgery.[11]
Interestingly, in this study, no significant associations were observed between the NEI VFQ-25 composite and VA for pre-surgery values or changes after cataract surgery among EVD survivors. While VA improved in the EVICT cohort overall, general health and composite scores did not improve after cataract surgery. The baseline NEI VFQ-25 values for general health were comparable to scores previously described for patients with cataracts; however, the composite scores in our population were generally lower than previously described cohorts with ocular disease.[13-15,18-23] Vision-related subscores of the NEI VFQ-25 that showed numerical improvements despite the lack of statistical significance were colour, distance and near visual acuities. There was a numerical decrease in the general vision score for participants who had pre-and post-cataract surgery assessments taken. Of note, besides these assessments of visual performance categories, one other category of the NEI VFQ-25 – peripheral vision – remained unchanged. Despite the lack of statistically significant improvements in the NEI VFQ-25 scores and a mix of numerical changes in the vision-related subscores, there was a numerical improvement in the overall general health score.
Questionnaires designed to assess visual function in resource-limited regions have been reported previously.[24,25] A questionnaire by van Dijk et al. with Malawian adults focused on activities of daily living and utilised locally appropriate questions.[24] Another vision specific-questionnaire was designed and applied in Timor-Leste and catered the questionnaire to the population’s activities, acknowledging that watching television or driving meant little in this study population compared to Westernised groups.[25] While comparing results between studies in different communities can be challenging when distinct questionnaires are utilised for different regional settings, the proper assessment of communities may require instruments that utilise statements and questions that apply directly to the study population.
Studies utilising the NEI VFQ-25 to assess visual disability in the setting of cataracts in various regions of the world often demonstrated improvements in general vision following cataract surgery, while increases in composite scores in the post-operative setting vary across differing geographies.[19,22,23] Fraser et al. reported that the first cataract surgery in patients with bilateral cataracts in Australia led to considerable improvements in general vision, near activities, distance activities and driving.[22] This study also demonstrated improvement in mental health categories, indicating that cataract surgery had some mental health benefits for patients in this population. Another study conducted on patients with retinitis pigmentosa in Japan found statistically significant improvements in all NEI VFQ-25 subscales except colour vision following bilateral cataract surgery.[19] Post-operative VA scores in the better eye were more strongly correlated with post-operative NEI VFQ-25 scores than for the worse eye.
Test-retest reliability of the NEI VFQ-25 was reported by To et al. when translated from English to Vietnamese.[21] Notably, there was a 100% non-response rate for questions related to ‘going out to see movies, plays or sporting events’ as it was irrelevant to this population. In addition, modifications were made to the questionnaire to adjust for cultural factors, such as replacing ‘driving’ with ‘riding a scooter or bicycle’ as driving was uncommon for the patients. The NEI VFQ-25 could not be validated following a study by Wan et al. in Chinese patients with bilateral cataracts. The authors reported that it was difficult to distinguish between participants with high visual function.[18] As this was a cross-sectional study, test-retest reliability was not assessed.
Study limitations
Several factors may have contributed to the lack of statistically significant improvement in NEI VFQ-25 scores. First, the mean baseline VA was poorer than logMAR VA 2.0 (i.e., Snellen VA 20/200 or Counting fingers at 2 feet), and 60% of individuals had a logMAR VA of 3.0 (i.e., Hand motions vision). It may have been impossible for these participants to improve their vision significantly, given poor baseline vision. In addition, EVD survivors underwent cataract surgery on their worst eye. They may have had sufficient vision in the fellow eye to compensate for visual loss in the study eye before cataract surgery. Specifically, baseline VA in the fellow eye for participants undergoing cataract surgery was logMAR 0.159, approximating Snellen VA of 20/30–20/40, which does not currently meet World Health Organisation criteria for mild vision impairment.[26] The NEI VFQ-25 is validated for use in English and was administered to participants by a translator. Therefore, the NEI VFQ-25 outcomes may have been impacted by language barriers, as medical interpretation was heavily relied on during the NEI VFQ-25 interview. Unfortunately, there are no other vision-related QoL assessments that have been validated for use in this population.
Only two EVD survivors answered the NEI VFQ-25 questions related to driving, as most participants in this study did not drive. Some items, such as the impact on driving, are not as applicable to people living in some areas of Sierra Leone as they might be for people living in a suburb of a United States city. Another limitation of this study is the small sample size, and the unique Sierra Leonean population may not be generalizable to other African populations or more broadly. EVD outbreaks have typically occurred in specific regions of Africa (e.g., Western Africa, DRC) that have not only widely varying host genetic backgrounds but also present unique sociocultural contexts and economic challenges. The NEI VFQ-25 was initially developed during a series of focus groups with patients with age-related cataracts, glaucoma, age-related macular degeneration, diabetic retinopathy or cytomegalovirus (CMV) retinitis. While the NEI VFQ-25 was developed in patients with potentially comparable conditions, including CMV retinitis (i.e., infectious posterior uveitis) and age-related cataracts, its utility has not been specifically validated in the context of visual impairment related to emerging infectious diseases such as EVD. Given the development of the NEI VFQ-25 for distinct diseases previously and sociocultural and economic contexts, future work could focus on further development and validation of instruments to quantify the impact of ocular disability on QoL in resource-limited communities of emerging diseases.
CONCLUSION
As the number of at-risk survivors continues to increase with periodic filovirus disease outbreaks, managing uveitis, post-uveitic cataract and other clinical sequelae in EVD survivors remains a significant challenge that is unlikely to decrease. While cataract surgery improved vision for EVD survivors in the EVICT study, significant changes were not observed in NEI VFQ-25 scores in general health and subscales. Potential limitations of the questionnaire include its applicability for resource-constrained environments and sociocultural contexts and better eye dominance limiting improvement seen through NEI VFQ-25 assessments. Specific questionnaires are needed to account for the unique quality-of-life measures in these communities, which could allow a more accurate representation of the impact of visual disability on the QoL in EVD survivors.
Acknowledgement
We gratefully thank the Ebola survivors in the EVICT study and their families for accompanying them on their journey to the eye clinic and through the ophthalmic assessments and treatment. This material was presented at the American Uveitis Fall Meeting in October 2022 in Chicago, IL.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
This project was supported by the National Eye Institute/ National Institutes of Health core grant P30-EY06360 (Department of Ophthalmology, Emory University School of Medicine), National Eye Institute, National Institutes of Health R01 EY029594 (Yeh) and K23 EY030158 (Shantha). Funding support was also provided through an Unrestricted Grant from Research to Prevent Blindness (Emory Eye Center, Emory University School of Medicine), the Marcus Foundation Combating Childhood Illness Seed Grant, Emory Global Health Institute and the Stanley M. Truhlsen Family Foundation, Inc. The funding organisation had no role in the design or conduct of this research. This work was further supported in part by federal funds from the National Cancer Institute and National Institutes of Health (NIH) under contract (75N91019D00024, Task Order no. 75N91019F00130) (IC). The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services of the institutions and companies affiliated with the authors, nor does mention of trade names, commercial products or organisations imply endorsement by the U.S. Government.
References
- An update on ocular complications of Ebola virus disease. Curr Opin Ophthalmol. 2017;28:600.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical manifestations and pathogenesis of uveitis in Ebola virus disease survivors. Ocul Immunol Inflamm. 2018;26:1128-34.
- [CrossRef] [PubMed] [Google Scholar]
- History of Ebola Virus Disease Outbreaks. 2022. Georgia, United States: Centers for Disease Control and Prevention (CDC); Available from: https://www.cdc.gov/vhf/ebola/history/chronology.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvhf%2Febola%2Foutbreaks%2Fhistory%2Fchronology.html#anchor_1526565058132 [Last accessed on 2022 Nov 23]
- [Google Scholar]
- Ophthalmic manifestations and causes of vision impairment in Ebola virus disease survivors in Monrovia, Liberia. Ophthalmology. 2017;124:170-7.
- [CrossRef] [PubMed] [Google Scholar]
- A longitudinal study of Ebola sequelae in Liberia. N Engl J Med. 2019;380:924-34.
- [CrossRef] [PubMed] [Google Scholar]
- Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. 2013;23:705-17.
- [CrossRef] [PubMed] [Google Scholar]
- Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332-6.
- [CrossRef] [PubMed] [Google Scholar]
- Ebola virus persistence in ocular tissues and fluids (EVICT) study: Reverse transcription-polymerase chain reaction and cataract surgery outcomes of ebola survivors in Sierra Leone. EBioMedicine. 2018;30:217-34.
- [CrossRef] [PubMed] [Google Scholar]
- Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med. 2015;372:2423-7.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety outcomes of cataract surgery in survivors of Ebola virus disease: 12-month results from the PREVAIL VII study. Transl Vis Sci Technol. 2021;10:32.
- [CrossRef] [PubMed] [Google Scholar]
- Visual acuity measurements. J Cataract Refract Surg. 2004;30:287-90.
- [CrossRef] [PubMed] [Google Scholar]
- The National Eye Institute 25-Item Visual Function Questionnaire (VFQ-25) Scoring Algorithm. 2000. Available from: https://www.nei.nih.gov/sites/default/files/2019-06/manual_cm2000.pdf [Last accessed on 2021 Dec 13]
- [Google Scholar]
- Development of the 25-list-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050-8.
- [CrossRef] [PubMed] [Google Scholar]
- The NEI VFQ-25 vision-related quality of life and prevalence of eye disease in a working population. Graefes Arch Clin Exp Ophthalmol. 2010;248:85-92.
- [CrossRef] [PubMed] [Google Scholar]
- Responsiveness of vision-specific and general quality of life metrics to ocular and systemic events in patients with uveitis. Ophthalmology. 2020;127:1710-8.
- [CrossRef] [PubMed] [Google Scholar]
- Associations among visual acuity and vision-and health-related quality of life among patients in the multicenter uveitis steroid treatment trial. Invest Ophthalmol Vis Sci. 2012;53:1169-76.
- [CrossRef] [PubMed] [Google Scholar]
- Validation and comparison of the National Eye Institute Visual Functioning Questionnaire-25 (NEI VFQ-25) and the Visual Function Index-14 (VF-14) in patients with cataracts: A multicentre study. Acta Ophthalmol. 2021;99:e480-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of cataract surgery on vision-related quality of life in patients with retinitis pigmentosa and the predictive factors of quality of life improvement. Biomed Res Int. 2021;2021:3846867.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with vision-related quality of life among the adult population living in Nagorno Karabagh. Public Health. 2017;153:137-46.
- [CrossRef] [PubMed] [Google Scholar]
- Assessing the test-retest repeatability of the Vietnamese version of the National Eye Institute 25-item Visual Function Questionnaire among bilateral cataract patients for a Vietnamese population. Australas J Ageing. 2014;33:E7-10.
- [CrossRef] [PubMed] [Google Scholar]
- Vision, quality of life and depressive symptoms after first eye cataract surgery. Psychogeriatrics. 2013;13:237-43.
- [CrossRef] [PubMed] [Google Scholar]
- Rejuvenation effects of cataract surgery with ultraviolet blocking intra-ocular lens on circadian rhythm and gait speed. Rejuvenation Res. 2014;17:359-65.
- [CrossRef] [PubMed] [Google Scholar]
- Creation and testing of a practical visual function assessment for use in Africa: Correlation with visual acuity, contrast sensitivity, and near vision in Malawian adults. Br J Ophthalmol. 1999;83:792-5.
- [CrossRef] [PubMed] [Google Scholar]
- Development and validation of a vision-specific quality-of-life questionnaire for Timor-Leste. Invest ophthalmol Vis Sci. 2008;49:4284-9.
- [CrossRef] [PubMed] [Google Scholar]
- Blindness and Vision Impairment. 2021. Geneva: World Health Organization; Available from: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment [Last accessed on 2022 Feb 03]
- [Google Scholar]







