Translate this page into:
Comparison of endothelial cell loss in Viscoless phacoemulsification versus soft shell technique of phacoemulsification: A retrospective study
-
Received: ,
Accepted: ,
How to cite this article: Mulchandani AV, Desai AU, Kaur G, Modi R. Comparison of endothelial cell loss in Viscoless phacoemulsification versus soft shell technique of phacoemulsification: A retrospective study. Glob J Cataract Surg Res Ophthalmol. doi: 10.25259/GJCSRO_1_2024
Abstract
Objectives:
The purpose of this study was to compare the consequences of soft shell and viscoless technique on corneal endothelial cells (CECs) in cataract patients after phacoemulsification.
Materials and Methods:
This is a retrospective study. A total of 206 eyes of 139 patients (75 males and 64 females) who underwent phacoemulsification surgery during the period from September 2019 to December 2019 were enrolled in the study. The patients who underwent phacoemulsification were divided into two groups depending on the type of surgery performed, that is, viscoless or soft shell technique. The pre-operative and post-operative records were compared. The primary outcome measure was endothelial cell count (ECC) measured at baseline and postoperatively. The differences between the two groups were analysed statistically. The study was done at Sohum Eye Care Centre, Mumbai, India.
Results:
No statistically significant differences were observed between the two groups in terms of age, gender, diabetic status and cumulative dissipated energy (CDE), as P > 0.05. The inter-group mean difference between CDE did not differ significantly (P = 0.0626 and 0.1075, for 1 and 3 months inter-group comparison) between the two groups. However, a statistically significant mean difference of 99.9516 cells/mm2 (P < 0.0001), 116.4146 cells/mm2 (P = 0.0031) after one month and 90.4468 cells/mm2 (P = 0.0051), 145.2895 cells/mm2 (P < 0.0001) 3 months of surgery, between pre- and post-operative ECC, was observed in group 1 and group 2, respectively. The values of Pearson’s correlation (r = −0.3 to +0.3) suggested a very weak and non-significant strength of association of endothelial cell loss with age, gender, diabetic status and CDE postoperatively. Intergroup post-operative endothelial cell loss did not show any significant difference after 1 (P = 0.0644) and three months (P = 0.052) of phacoemulsification.
Conclusions:
Viscoless phacoemulsification surgery may be recommended in appropriate patients to avoid the side effects of ophthalmic viscosurgical devices.
Keywords
Phacoemulsification
Viscoless
Soft-shell
Conventional phacoemulsification
INTRODUCTION
Corneal endothelial cells (CECs) play a very important role in regulating corneal hydration through their pump mechanism. Their loss below the critical number (<500 cells/mm2) may progress to corneal oedema and decrease corneal clarity with decreased visual acuity as a consequence.[1] Nature has provided a sufficient number of endothelial cells (2500 cells/mm2) to ensure corneal integrity. The number of cells declines at different levels with age, as measured in various studies. Approximately 0.6% decline per year is widely agreed.[2-4] These cells cannot normally replicate, the loss of which is overcome by migration, enlargement and increased cell heterogeneity.[1] This corneal decompensation is uncommon but probably the vision-threatening consequence of phacoemulsification surgery.[5-10] However, the risk of CEC loss may be reduced by ensuring adequate surgical space during phacoemulsification.[11] Anatomical and surgical aspects, such as sufficient anterior chamber (AC) depth, are therefore vital in protecting such cells from the structural and physiological disruption that can happen throughout the surgery.[12]
Ophthalmic viscosurgical devices (OVDs) are used in cataract surgery to maintain the AC and also to protect the endothelium.[13] On the contrary, some OVDs were shown to enhance heat generation and, thus, induce endothelial cell loss.[14] OVDs may also trigger elevated intraocular pressure (IOP) and inflammation in the immediate post-operative period, which may lead to greater loss of endothelial cells.[15] Furthermore, additional efforts are required to remove OVD from the AC, which may cause significant damage to the endothelial cells.[16]
Balanced salt solution (BSS) is suitable for intraocular irrigation, which helps to maintain the natural condition of the eye during surgery and also decreases endothelial cell loss. It can avoid the need for an OVD, consequently preventing the inherent risks in OVDs.[17,18] The results of the technique seem promising, but the data are still insufficient.[16,19-22]
This study aimed to evaluate the effect of BSS-assisted viscoless phacoemulsification on post-operative CEC density and to compare this data with the soft shell technique.
MATERIALS AND METHODS
This is a retrospective study including patients who underwent cataract surgery at Sohum Eye Care Centre, Mumbai, Maharashtra, India.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.[23]
The Ethical committee approval was obtained.
A total of 206 eyes of 139 patients (75 males and 64 females) who underwent phacoemulsification surgery during the period from September 2019 to December 2019 were enrolled in the study.
The eyes operated on were divided into two groups, that is, group 1 and group 2, for a post-operative analysis of 1 and 3 months of endothelial cell count (ECC), as shown in Figure 1. The patients in whom the soft shell technique was used were put in group 1, while the patients in whom the viscoless technique was used were put in group 2. In all cases, one month of post-operative ECC data was available and was studied; however, three months of post-operative data were available only in 85 cases.
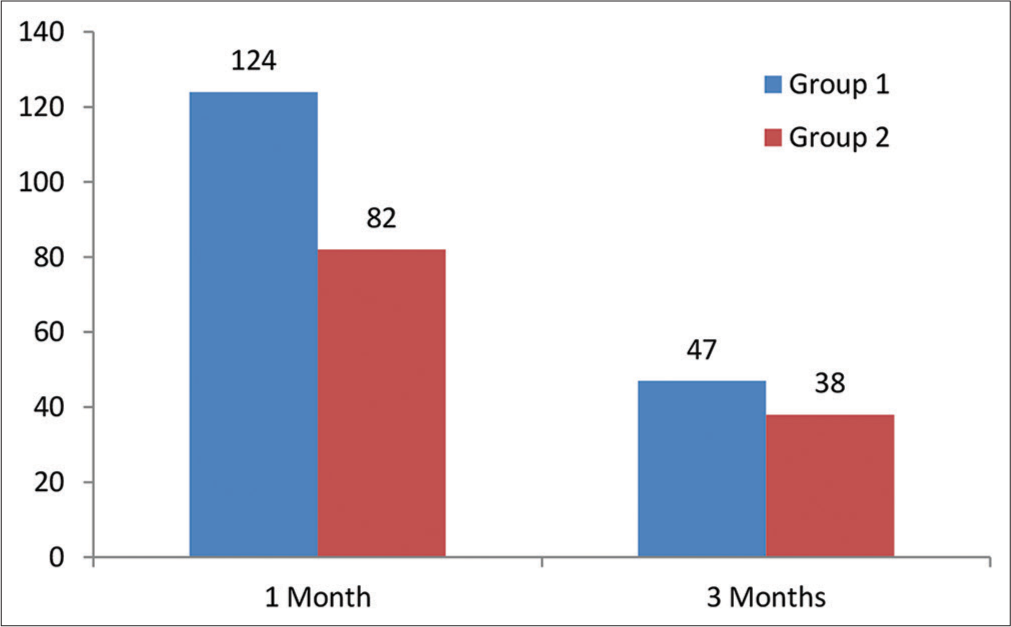
- Number of eyes involved in group 1 and group 2.
The exclusion criteria were as follows: (1) Nuclear sclerosis (NS) Grade 5 and beyond, complicated cataract, subluxated cataract; (2) pre-operative ECC <1500 cells/mm2; (3) retinal and other ocular comorbidities such as pseudoexfoliation, glaucoma and uveitis; (4) pupil size <7.5 mm after dilatation; (5) any intraoperative complications; (6) types of cataract other than age-related (e.g., secondary or congenital); (7) previous ocular surgery; (8) previous corneal disease and (9) any condition that impeded corneal evaluation by specular microscopy and pachymetry or follow-up.
Records of pre-operative evaluation of the patients were obtained, which included the measurement of uncorrected visual acuity, best-corrected visual acuity, an examination of the anterior segment of the eye under slit-lamp bio-microscope, central corneal thickness and fundus evaluation through a dilated pupil, grading (0–5+) of the NS (a combination of opalescence and yellowing) according to Lens Opacities Classification System III classification and IOP with Goldmann applanation tonometer and non-contact tonometer in all the patients along with endothelial analysis in an area of 0.25 × 0.54 mm2 of the central corneal endothelium performed automatically by a non-contact computer-assisted specular microscope (EM-4000 Tomey, USA).
The patients who were operated by a single surgeon were chosen for the study. All surgeries were performed under topical anaesthesia. Phacoemulsification was performed using the Centurion Vision System (Alcon Laboratories, Inc.) using a standardised surgical technique for soft cataract up to NS Grade 1, vacuum aspiration or chip and flip method was performed depending on the case; Grade 2 and beyond, direct chop was done. All surgeries were performed through a 2.3 mm incision using a balanced phaco tip and sleeve (Alcon Laboratories, Inc.). Anatomic landmarks of the eye were obtained using the Verion Image Guided System (Alcon Laboratories, Inc.).
Following capsulorhexis hydrodissection, the nucleus was managed by the techniques mentioned above. Phacoemulsification was done at an intraoperative IOP of 26 mmHg, 459 mmHg vacuum (max.) and aspiration flow rate of 26 cm3/min and 60% torsional phacoemulsification with IP setting at 90% occlusion. Irrigation aspiration was done at an intraoperative IOP of 40 mmHg, and intraocular lens (IOL) insertion in group 2 was done under I/A, while it was done viscoelastics in group 1.
The following steps were followed during the surgery:
-
Soft shell technique
Two VERION-guided side port incisions of 1.3 mm were made.
Viscoat was injected through one side port, followed by provisc, after which the main incision (2.3 mm) was made.
Verion-guided capsulorhexis was performed using 26G cystitome/capsulorhexis forceps. Hydrodissection was performed with a hydrodissection cannula until a complete wave was observed.
Phacoemulsification was performed without any difference from a standard procedure. (For soft cataract up to NS grade 1, vacuum aspiration or chip and flip method was performed depending upon the case; for Grade 2 and beyond, direct chop was done.)
Alcon foldable one-piece IOL (models SA60AT, SN60WF, SNAT3-6, TFTN) with injector and D cartridge were used. The IOLs were loaded using sodium hyaluronate (provisc) in the cartridge.
-
Viscoless technique[24]
Two VERION-guided side port incisions of 1.3 mm were made.
An irrigation cannula was introduced through one side port; the main incision (2.3 mm) was made. The size of the side port blade and the irrigation cannula must be the same to avoid leakage through the corneal incision.
Verion-guided capsulorhexis was performed using 26G cystitome/side port capsulorhexis forceps under continuous irrigation. Hydrodissection was performed with the same irrigation cannula or a hydrodissection cannula until a complete wave was observed.
Phacoemulsification was performed without any difference from a standard procedure (For soft cataract up to NS grade 1, vacuum aspiration or chip and flip method was performed depending upon the case; for Grade 2 and beyond, direct chop was done).
Alcon foldable one-piece IOL (models SA60AT, SN60WF, SNAT3-6, TFTN) with injector and D cartridge were used. The IOLs were loaded using saline in the cartridge. Records of specular microscopy, which was done for all patients at one month and three months postoperatively, were analysed.
Statistical analyses were performed using MATLAB software (Version R2013a; MathWorks, Natick, MA, USA). The mean differences between independent samples for the two groups were assessed using the independent sample t-test. A Chi-square test was used to compare the proportions of a categorical outcome according to different independent groups. Intergroup and intragroup comparisons were analysed by means of the Mann–Whitney U-test and paired t-test, respectively. Pearson’s correlation coefficient was used to measure the strength of the linear association between two variables. The Welch test was applied for intergroup comparison when the groups had unequal variance. The significance level adopted was 5% (P < 0.05).
RESULTS
Age group
A statistical analysis using an independent sample t-test indicated that there was no statistically significant difference in the mean age of group 1 participants relative to group 2 participants as (t(204) = 0.663, P = 0.5079 and t(83) = −1.727, P = 0.088 for the participants involved in analysis of 1 and 3 months, respectively.
Gender distribution
The collected data represented that the incidence of cataract was higher among women, as shown in Figure 2. However, the Chi-square test statistics demonstrated that the participants involved in groups 1 and 2 did not differ by gender as χ 2 = 0.542, P = 0.4616 (for one-month postoperative data analysis) and χ2 = 0.549 P = 0.4616, 0.441 (for three months post-operative data analysis).
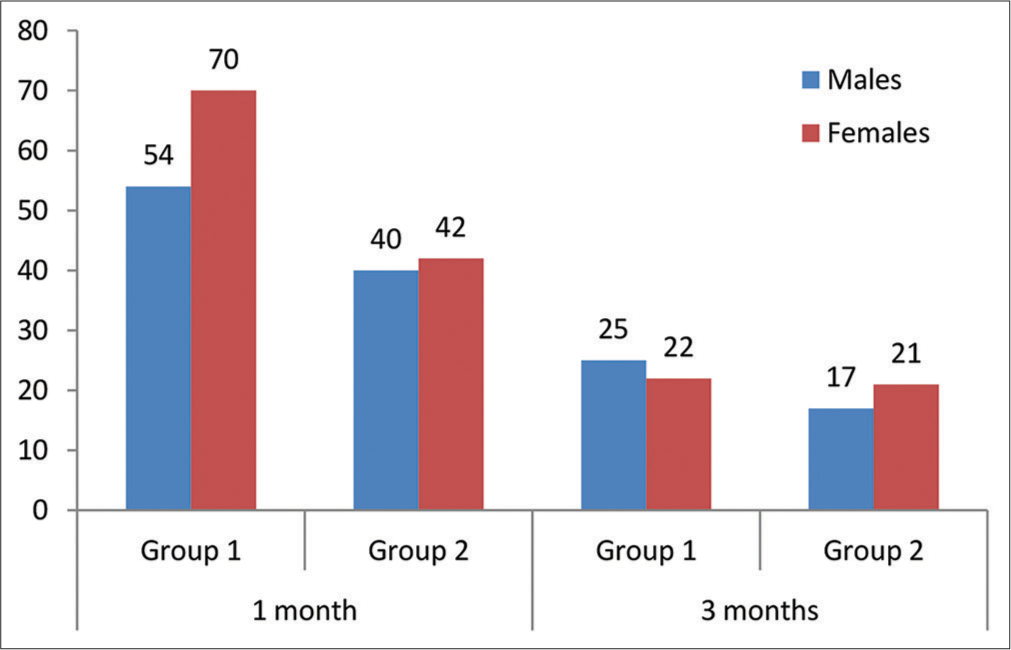
- Gender distribution between groups.
Diabetic status
It was observed that the number of non-diabetic patients in each group was higher as compared to diabetic, as presented in Figure 3. Nevertheless, the results of the Chi-square test showed that the participants of groups 1 and 2 did not differ by their diabetic status as χ 2 = 2.003, P = 0.1569, for one-month post-operative data analysis and χ 2 = 1.073, P = 0.3002, for three months post-operative data analysis.
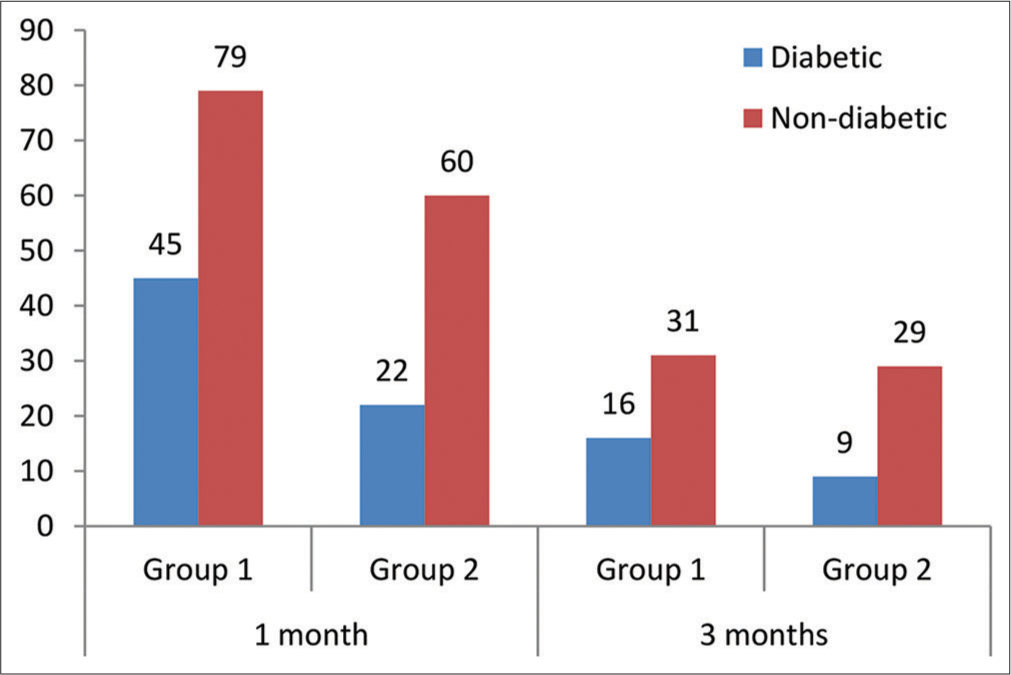
- Comparison of diabetic status of the participants enrolled in the study.
Cumulative dissipated energy (CDE)
The CDE applied in group 1 was compared to group 2 using the Welch test, as the F test for equality of variance failed (P < 0.001, P = 0.001, for 1 and 3 months CDE data). The mean CDE applied did not differ in the two groups by the type of AC solution used during surgery as t(204)=1.872, P = 0.0626 (1 month postoperatively) and t(76.7)=1.629, P = 0.1075 (3 months postoperatively).
Comparison between pre- and post-operative ECC
Pre-operative versus one month post-operative ECC analysis
In group 1, the mean pre-operative and one-month post-operative ECC was 2533.9194 cells/mm2 and 2433.9677 cells/mm2, respectively.
In group 2, the mean pre-operative and one-month post-operative ECC was 2605.8659 cells/mm2 and 2489.4512 cells/mm2, respectively.
A paired t-test was performed to assess if there was a statistically significant mean difference in pre-operative and one-month post-operative ECC values. A statistically significant (P < 0.0001 and 0.0031 for groups 1 and 2, respectively) mean difference (99.9516 cells/mm2 and 116.4146 cells/mm2 in groups 1 and 2, respectively) between pre-operative and one month post-operative ECC value was observed, as indicated by test statistics given in Table 1.
| Test statistics | Group 1 | Group 2 |
|---|---|---|
| Mean difference | 99.9516 | 116.4146 |
| The standard deviation of differences | 254.0327 | 345.4597 |
| Standard error of the mean difference | 22.8128 | 38.1496 |
| 95% CI of difference | 54.7951–145.1082 | 40.5088–192.3205 |
| Test statistic t | 4.381 | 3.052 |
| DOF | 123 | 81 |
| Two-tailed probability (P) |
<0.0001 | 0.0031 |
| Reduction in ECD (%) | 3.94 | 4.47 |
ECC: Endothelial cell count, DOF: Degree of freedom, CI: Confidence interval, ECD: Endothelial cell density
Pre-operative versus three months post-operative ECC analysis
In group 1, the mean pre-operative and three months postoperative ECC was 2553.9787 cells/mm2 and 2463.5319 cells/mm2, respectively.
In group 2, the mean pre-operative and one-month postoperative ECC was 2594.0526 cells/mm2 and 2448.7632 cells/mm2, respectively.
The results of the paired t-test revealed a statistically significant (P = 0.0051 and <0.0001 in groups 1 and 2, respectively) mean difference (90.4468 cells/mm2 and 145.2895 cells/mm2 in groups 1 and 2, respectively) between pre- and three month post-operative ECC, as shown in Table 2.
| Test statistics | Group 1 | Group 2 |
|---|---|---|
| Mean difference | 90.4468 | 145.2895 |
| The standard deviation of differences | 210.6212 | 171.2180 |
| Standard error of the mean difference | 30.7223 | 27.7752 |
| 95% CI of difference | 28.6061–152.2875 | 89.0115–201.5674 |
| Test statistic t | 2.944 | 5.231 |
| DOF | 46 | 37 |
| Two-tailed probability (P) |
0.0051 | <0.0001 |
| Reduction in ECD (%) | 3.54 | 5.6 |
ECC: Endothelial cell count, DOF: Degree of freedom, CI: Confidence interval, ECD: Endothelial cell density
Correlation of age, gender and diabetic status with postoperative endothelial cell loss
Pearson’s correlation coefficients obtained between endothelial cell loss (1 and 3 months postoperatively) and patient’s characteristics, including age, gender, diabetic status and CDE applied during surgery, showed weak and statistically non-significant positive or negative correlation (r- value ranging from −0.3 to +0.3). Furthermore, all correlations were found to be not statistically significant.
Intergroup comparison of postoperative endothelial cell loss
Post-operative endothelial cell loss in group 1 was compared to group 2, as presented in Table 3. While the endothelial cell loss in group 2 was of higher average rank (112.9451, 48.7895, for 1 and 3 months post-operative data, respectively) than the average rank of group 1 (97.2540, 38.3191, for 1 and 3 months post-operative data, respectively), the difference was not statistically significant (P = 0.0644, 0.052, for 1 and 3 months post-operative data, respectively).
| Test statistics | One month post-operative (Group 1 vs. Group 2) |
3 months post-operative (Group 1 vs. Group 2) |
|---|---|---|
| The average rank of the first group |
97.254 | 38.3191 |
| The average rank of the second group |
112.9451 | 48.7895 |
| Mann–Whitney U | 4309.5 | 673 |
| Test statistic Z (corrected for ties) |
−1.849 | −1.945 |
| Two-tailed probability (P) | 0.0644 | 0.052 |
DISCUSSION
OVDs offer many surgical benefits in modern cataract surgery. However, in addition to surgical advantages like preserving AC stability during capsulorhexis, increasing the size of the pupils in small pupils sustaining the iris in floppy iris syndrome, decreasing the risk of the rupture of the posterior capsule and promoting IOL implantation,[13] post-operative risks associated with OVDs have recently started to be addressed, and there is growing interest in solving such challenges among cataract surgeons.
One of the controversial concerns is the preservation of endothelial cells. This has been well-documented that OVDs shield endothelial cells from contact with intraocular structures, IOLs and tools.[13,25] The elimination of free radicals throughout phacoemulsification is also another detrimental effect of OVDs.[15] In addition, OVDs may minimize the wrinkle of the cornea and the mechanical pressure on the endothelial cells during the application of the IOL injector.[21] On the other hand, OVDs have been reported to increase thermal damage, resulting in a loss of endothelial cells.[26] for which the reason is that if there is no flow of fluid across the phaco tip as a consequence of the viscoelastic substance, the temperature of the tip may rise and the cornea may be burned, causing damage to the endothelial cells.[13] Moreover, prolonged I/A to fully remove the applied viscoelastic for IOL implantation increases the surgical time and fluid flow and may, therefore, impact the properties of the endothelial cells.[13,21] In addition, the remaining OVD in the eye may stimulate IOP surges and inflammation immediately after cataract surgery, which may cause more damage to the endothelial cells.[26,27]
A further potential post-operative issue associated with OVDs is the early phase of capsular block syndrome if some of the viscoelastic remains behind the IOL. It was associated with a distended bag that triggered the anterior shift of the IOL and a myopic change.[28] No cases of capsular block syndrome or myopic change were found in either group in this study.
Additional surgical time and increased I/A period can increase IOP and fluid flow through the chamber, which could affect endothelial cells.[21] A potential benefit of shorter surgical duration is that the prospect of performing a larger number of surgeries will lead to a more cost-effective use of the operation room. Furthermore, the purchasing cost of BSS is lower than OVDs, which can also lower the cost of surgery.
In view of the above knowledge, the present study compared changes in endothelial cells using viscoless phacoemulsification with the soft shell technique.
The risk of endothelial cell damage increases with a high nucleus grade, advanced age, long phacoemulsification time, high CDE or ultrasound energy, small pupil diameter, capsular rupture or vitreous loss during surgery, short axial length and diabetes.[29,30]
The present study groups were fairly comparable in age, gender, diabetic status and CDE applied during surgery. In addition, we have excluded pupils of <7.5 mm in diameter. In other words, in addition to controlling confounding variables through randomisation, we further strengthened the internal validity of the study by controlling these confounders through statistical procedures. Furthermore, studies have demonstrated increased endothelial cell loss with elevated rates of NS. Therefore, in the present study, all cataracts beyond the grade 5 nucleus were omitted.
In this investigation, all surgeries were performed by a single surgeon and using a single phaco machine. Thus, the incision size and phacoemulsification technique were equivalent for both groups.
Our findings showed that the endothelial cell loss was 3.94% and 3.54% in the viscoless group and 4.47% and 5.6% in the conventional soft shell group at the end of 1 month and three months, respectively. The loss of endothelial cells was comparable to the results of other studies. Studeny et al.[20] reported CEC loss of 9.76% and 10.7% in the hydroimplantation category and 9.07% and 9.13% in the OVD category at one month and three months, respectively, following the surgery.
Postoperatively, both groups had a statistically significant decrease in endothelial cell density. Overall, it is widely accepted that endothelial cell loss occurs as an inevitable consequence of cataract surgery. A decrease in the endothelial-cell count in both groups postoperatively may be due to multiple factors, such as surgical technique and skills, and the severity of cataract.
Furthermore, no significant correlation between patient demographics, CDE applied, and endothelial cell count was seen in this study.
Post-operative endothelial cell loss caused by viscoless phacoemulsification surgery, when compared to the soft shell technique, a non-significant difference (P = 0.0644 and 0.052 after one month and three months, respectively) demonstrates that viscoelastic devices may not be necessary at all.
The limitations of the present study are as follows:
Small sample size and short follow-up time.
The BSS-assisted or viscoless phacoemulsification may not be appropriate for hard cataracts.
In our hands, insertion of three-piece IOL using a viscoless technique is difficult and hence is not performed.
The proposed technique is not appropriate for individuals with endothelial corneal disease, PEX disorder, severe cataracts and/or a history of prior vitreoretinal surgery. However, in these situations, the standard phacoemulsification technique may also increase the surgical risk.
Sequential endothelial findings after intraocular surgery have shown that endothelial cells are in a state of transition for a long post-operative duration with a gradual decline in cell density. Thus, a longer period of follow-up is required as endothelial cell loss is known to occur for a long time after cataract surgery.
CONCLUSION
The viscoless phacoemulsification surgery is a reliable technique. Our findings indicate that the final long-term outcomes in the corneal endothelium may be the same in either group. However, a larger database and probably more multicentric studies are required to substantiate our study.
Ethical approval
The research/study was approved by the Institutional Review Board at Bhaktivedanta Hospital and Research Institute, number BMR/16/2021, dated 04/05/2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Kanski’s clinical ophthalmology: A systematic approach (9th ed). China: Elsevier; 2016.
- [Google Scholar]
- Age-related changes of corneal endothelium in normal eyes with a non-contact specular microscope. J Emmetropia. 2010;1:132-9.
- [Google Scholar]
- Depth and age-dependent distribution of keratocytes in healthy human corneas: A study using scanning-slit confocal microscopy in vivo. J Cataract Refract Surg. 2002;28:611-6.
- [CrossRef] [PubMed] [Google Scholar]
- Age-related differences in the normal human cornea: A laser scanning in vivo confocal microscopy study. Br J Ophthalmol. 2007;91:1165-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of incision size and site on corneal endothelial changes in cataract surgery. J Cataract Refract Surg. 2002;28:118-25.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for endothelial cell loss after phacoemulsification surgery by a junior resident. J Cataract Refract Surg. 2004;30:839-43.
- [CrossRef] [PubMed] [Google Scholar]
- Endothelial damage with cataract surgery techniques. J Cataract Refract Surg. 1998;24:951-5.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term endothelial cell loss following phacoemulsification through a temporal clear corneal incision. J Cataract Refract Surg. 1996;22:63-71.
- [CrossRef] [PubMed] [Google Scholar]
- Corneal endothelium evaluation after phacoemulsification with continuous anterior chamber infusion. Cornea. 2005;24:278-82.
- [CrossRef] [PubMed] [Google Scholar]
- Endothelial cell loss after phacoemulsification: Relation to preoperative and intraoperative parameters. J Cataract Refract Surg. 2000;26:727-32.
- [CrossRef] [PubMed] [Google Scholar]
- Endothelial cell loss after phacomulsification according to different anterior chamber depths. J Opthalmol. 2015;2015:210716.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of anterior chamber depth and axial length on corneal endothelial cell density after phacoemulsification. Pak J Med Sci. 2019;35:200-4.
- [CrossRef] [PubMed] [Google Scholar]
- Ophthalmic viscosurgical devices. Curr Opin Opthalmol. 2008;19:50-4.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of incisional friction and ophthalmic viscosurgical devices on the heat generation of ultrasound during cataract surgery. J Cataract Refract Surg. 2006;32:1222-6.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative tissue damage after phacoemulsifiction: Influence of ophthalmic viscosurgical devices. J Cataract Refract Surg. 2004;30:424-7.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of corneal endothelial cell loss during phacoemulsification using continuous anterior chamber infusion versus those using ophthalmic viscosurgical device: Randomised controlled trial. Indian J Opthalmol. 2009;57:99-103.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/018469s052lbl.pdf [Last accessed on 2024 Jan 02]
- Effect of irrigating solution and irrigating temperature on the cornea and pupil during phacoemulsification. J Cataract Refract Surg. 2000;26:392-7.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroimplantation: Foldable intraocular lens implantation without an ophthalmic viscosurgical device. J Cataract Refract Surg. 2010;36:377-9.
- [CrossRef] [PubMed] [Google Scholar]
- Safety of hydroimplantation: A foldable intraocular lens implantation without the use of an ophthalmic viscosurgical device. Eur J Opthalmol. 2014;24:850-6.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in corneal endothelium cell characteristics after cataract surgery with an without use of viscoelastic substances during intraocular lens implantation. Clin Opthalmol. 2015;9:2073-80.
- [CrossRef] [PubMed] [Google Scholar]
- Balanced salt solution-assisted intraocular lens implantation in phacoemulsification surgery: Intraocular pressure and endothelial cell effects. Beyoglu Eye J. 2019;4:5-10.
- [CrossRef] [PubMed] [Google Scholar]
- World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. J Am Med Assoc. 2013;310:2191-4.
- [CrossRef] [PubMed] [Google Scholar]
- Phacoemulsification cataract surgery without viscoelastic substance. JOJ Ophthalmol. 2017;4:555646.
- [CrossRef] [Google Scholar]
- Anterior chamber maintainer versus viscoelastic material for intraocular lens implantation: Case-control study. J Cataract Refract Surg. 2001;277:711-4.
- [CrossRef] [PubMed] [Google Scholar]
- Results of a randomised controlled trial. Oxford Cataract Treatment and Evaluation Team (OCTET) Arch Opthalmol. 1986;104:1170-5.
- [CrossRef] [PubMed] [Google Scholar]
- Intraocular pressure rise after phacoemulsification with posterior chamber lens implantation: Effect of prophylactic medication, wound closure, and surgeon's experience. Br J Opthalmol. 1995;79:809-13.
- [CrossRef] [PubMed] [Google Scholar]
- New classification of capsular block syndrome. J Cataract Refract Surg. 1998;24:1230-4.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for corneal endothelial injury during phacoemulsification. J Cataract Refract Surg. 1996;22:1079-84.
- [CrossRef] [PubMed] [Google Scholar]
- Endothelial cell damage after cataract surgery: Divide-and-conquer versus phaco-chop technique. J Cataract Refract Surg. 2008;34:99-1000.
- [CrossRef] [PubMed] [Google Scholar]







