Translate this page into:
Prediction of effective lens position (ELP) and its changes in different monofocal intraocular lens (IOL’s)
*Corresponding author: Ramya Umarani, Department of Phacorefractive, Nethradhama Superspeciality Eye Hospital, Bengaluru, Karnataka, India. ramyaumarani21@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pereira S, Ganesh S, Umarani R, Sute SS. Prediction of effective lens position (ELP) and its changes in different monofocal intraocular lens (IOL’s). Glob J Cataract Surg Res Ophthalmol 2022;1:93-8.
Abstract
Objectives:
To evaluate effective lens position (ELP) is more accurately predicted by anterior chamber depth (ACD) alone or by ACD + ½ lens thickness (LT) and to compare the ELP and refractive outcome of different monofocal intraocular lens (IOLs) in patients undergoing phacoemulsification.
Materials and Methods:
A total of 122 eyes with senile cataract underwent phacoemulsification with three different types of IOL implantation. Biometry data were obtained by IOL master 700 (SS-OCT) and IOL power was calculated using Barrett Universal II formula. Two prediction formulae for ELP were compared, one with ACD + ½ LT and other with ACD alone; furthermore, comparison was done among three types of IOL. Mean prediction error was calculated for both methods and among three IOL groups.
Results:
In the study, predicted ELP according to ACD + ½ LT was 5.27 ± 0.27 and Mean pre-op ACD was 3.069 ± 0.349 mm, achieved ELP (post-op ACD) was 4.98 ± 0.47 mm and between the two; ACD + ½ LT is closer to achieved ELP. The difference between mean ACD + ½ LT (5.22) and achieved ELP (4.71) was 0.50 in ALCON ACRYSOF IQ (SN60WF) and difference of ACD + ½ LT (5.33) and achieved ELP (5.07) was 0.25 in J and J TECNIS 1 (ZCB00), while the difference of ACD + ½ LT (5.28) and achieved ELP (5.16) was 0.114 in ZEISS CT LUCIA (611P). Residual refraction predicted by IOL master 700 and achieved residual refraction at post-op 1 month was −0.15 ± 0.19 and −0.10 ± 0.30 in ACRYSOF IQ, was −0.11 ± 0.18 and −0.01 ± 0.20 in TECNIS 1 and was −0.10 ± 0.20 and + 0.396 ± 0.22 in ZEISS CT LUCIA, respectively.
Conclusion:
ELP may be better predicted by formulae ACD + ½ LT than ACD alone with mean differences of 0.29 and 1.92 in two prediction groups, respectively. ALCON ACRYSOF IQ (SN60WF) and J and J TECNIS 1 (ZCB00) group had myopic shifts of 0.10 and 0.11, respectively, while ZEISS CT LUCIA (611P) group had hyperopic shifts of 0.5002 which was statistically significant, as ALCON ACRYSOF IQ (SN60WF) and J and J TECNIS 1 (ZCB00) ELP is positioned more anterior and ZEISS CT LUCIA (611P) relatively posterior.
Keywords
Evaluate effective lens position (ELP)
Anterior chamber depth (ACD)
Monofocal intraocular lens (monofocal IOL)
INTRODUCTION
Refractive surprise after cataract surgery is an unpleasant situation both for patient and the treating surgeon. With recent innovations in phacoemulsification techniques, ocular biometry and intraocular lens (IOL) power prediction, cataract surgery has evolved into a refractive procedure. Patient expectations have increased nowadays, as everyone desires spectacle independence, imposing pressure on operating surgeons to deliver their best in achieving good refractive outcomes. Refractive outcome depends on the accurate power of the implanted IOL, which in turn depends on pre-operative biometry data, IOL power calculation formulae and manufacturer IOL power quality control.[1] In IOL power calculation formulae, corneal curvature, axial length (AL) and the postoperative position of the IOL implanted within the eye, referred to as the effective lens position (ELP) play an important role in the refractive outcome prediction.[2] ELP is the effective distance between the anterior surface of the cornea and the lens plane if the lens was infinitely thin.[3]
Each IOL power formula determines ELP utilising different theoretical models, thus choice of IOL power formula is critically important factor in achieving accurate postoperative refractive outcomes.[4] The fourth generation formulation, the Barrett Universal II is regarded as the more accurate and predictable among all the available formulas, it uses five inputs in the calculation algorithm: Anterior chamber depth (ACD), AL, keratometry (K), lens thickness (LT) and white-to-white distance (WTW).[5] The optimised A constant of the SRK/T formula was converted using the calculation tool of the Barrett Universal II formula to calculate the Constant value of the Barrett Universal II formula (Lens factor) which is based on Gaussian principles or ray tracing.[5] It takes into account the change in principal planes that occur with different IOL powers and has a unique theoretical model to predict the ELP, the Barrett universal. Across all AL ranges the LT s has additional accuracy in the prediction of the ELP.[6] Furthermore, important factor to be noted is ELP is affected by parameters like the preoperative capsule size, severity of the cataract, uneventful cataract surgery, capsular integrity, rhexis centration, bag size, postsurgical capsule contraction, IOL Power, IOL thickness thus accurate prediction is a challenging task.[7-9]
There are various methods by which ELP has been calculated previously. Mathematical formulae have been developed for best estimation of ELP using paraxial optics based on parameters of OCT Biometry and AS-OCT taking into consideration the intended postoperative refraction.[10-12] Dooley et al.[13] used K-independent method of predicting ELP and compared it with K-dependent method, meanwhile Tamaoki et al.[14] used multi-objective evolutionary algorithm to predict ELP and found that it is more accurate with less fluctuation when compared with stepwise multiple regression analysis. Olsen et al.[11] concluded the postoperative ACD was significantly correlated with and was predictable by a 5-variable regression method incorporating the pre-operative AL, ACD, K, LT and refraction as the most significant variables.
The purpose of this study was comparison of the two methods to determine which predicts ELP more accurately and to know change between predicted and achieved ELP in different types of IOLs.
MATERIALS AND METHODS
This prospective interventional, non-randomised study was approved by the institutional ethics committee of Nethradhama Super Speciality Eye Hospital, Bengaluru and abided by the tenets of the Declaration of Helsinki.
Inclusion criteria were patient willing for phacoemulsification and PCIOL implantation giving written informed consent with age 40–70 years having healthy eyes besides senile cataract up to grade NC3 as per LOCS III grading, corneal astigmatism <1.00 diopters (D), AL between 22.0 mm and 24.50 mm, IOL power between +17.0 and +26.00 D, uneventful surgery and assured follow-up. Exclusion criteria were patients with high myopia >6D and astigmatism >1D, irregular astigmatism due to corneal abnormalities, patients with corneal opacities, pseudoexfoliation syndrome, traumatic cataract and previous ocular surgery including refractive surgery, history of glaucoma, intraocular inflammation, neuro-ophthalmic and retinal disorders, immunocompromised state, on any systemic medication likely to affect wound healing such as corticosteroids or antimetabolites.
Preoperatively, all patients underwent complete ophthalmic examination which included measurement of uncorrected and corrected distant visual acuity with ETDRS charts at 4 m (Precision Vision, USA), manifest refraction, slit-lamp biomicroscopy, non-contact tonometry and dilated fundus examination. Biometric assessments were performed using a swept-source OCT-based optical biometer, the IOL Master-700 (Carl Zeiss Meditec, Jena, Germany), using the Barrett TK® (Total K) Universal II formula. All eyes were targeted for emmetropia. An optimised A-constant of 118.3 or lens factor constant as per the manufacturer was used for IOL power calculation.
Surgical technique
All the patients were operated on under topical anaesthesia (0.5% Proparacaine hydrochloride) by single surgeon and a single investigator performed all pre-operative tests. Phacoemulsification surgery was performed through a 2.8 mm temporal clear corneal incision and continuous curvilinear capsulorrhexis of 5.5 mm was made. A posterior chamber foldable single monofocal biconvex IOL ALCON ACRYSOF IQ (SN60WF)/J and J TECNIS 1 (ZCB00)/ZEISS CT LUCIA (611P) was implanted into the capsular bag. The power of the IOL implanted coincided with the power calculated preoperatively. Patients were counselled about post-operative medications and followed up on days 1, day 15 and 1 month. During each follow-up uncorrected and best corrected visual acuity by subjective refraction, IOP measurement and clinical examination was done.
Statistical methods
Statistical software: MS Excel, SPSS version 22 (IBM SPSS Statistics, Somers NY, USA) was used to analyse data. Analysis of variance was the test of significance to identify the mean difference between more than two groups for quantitative data. Paired t-test is the test of significance for paired data such as before and after surgery for quantitative data. P < 0.05 was considered statistically significant.
RESULTS
In this study, age distribution of subjects was in the range of 61–70 years, mean age of subjects was 66.55 ± 7.936 years and equal number of male and female patients (61 each) who complied with study criteria were recruited. The mean AL was 23.31 mm ± 0.68 mm, Mean IOL power implanted was 21.76D ± 2.02 D. Among 122 eyes 40 eyes had ALCON ACRYSOF IQ (SN60WF) IOL, 41 had J and J TECNIS 1 (ZCB00) IOL and 41 had ZEISS CT LUCIA (611P) IOL implanted.
In the study predicted ELP was 5.28 ± 0.28 and achieved ELP (post op ACD) was 4.98 ± 0.47 and by considering ACD + ½ LT, there was significant difference between them (P < 0.001). The mean pre Op ACD was 3.06 ± 0.34 mm and achieved ELP (post-op ACD) was 4.98 ± 0.47 mm and significant increase in post-op ACD compared to pre-op ACD was noted (P < 0.001). In comparison ACD + ½ LT is closer to achieved ELP [Table 1 and Figure 1].
| Parameter (n=122) | Mean±SD | P-value |
|---|---|---|
| ACD+½ LT | 5.279±0.276 | <0.001 |
| Pre-op ACD | 3.069±0.349 | |
| Achieved ELP | 4.985±0.472 |
ACD: Anterior chamber depth, LT: Lens thickness, ELP: Effective lens position
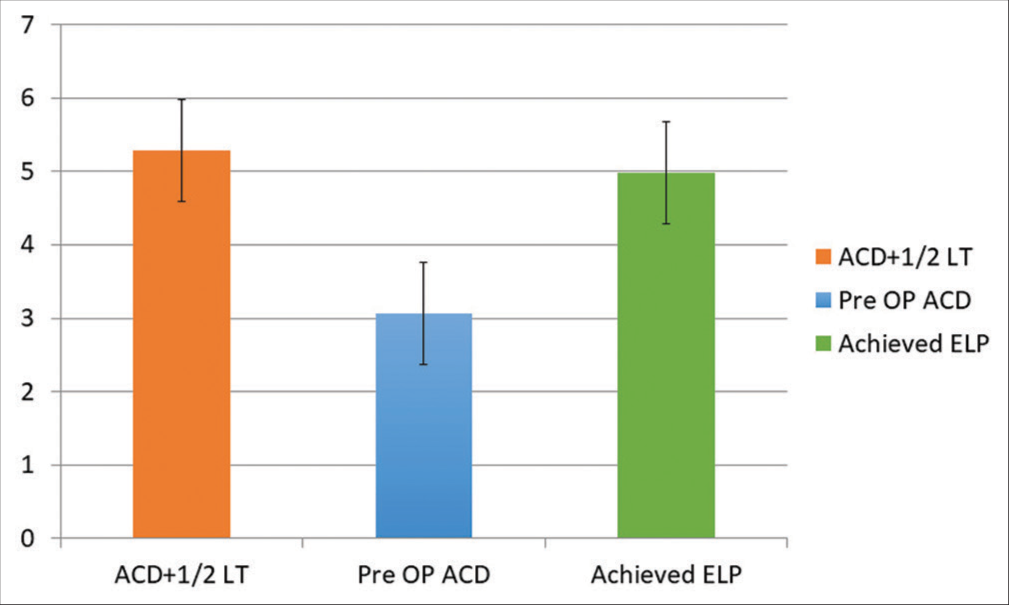
- Comparison of ACD + ½ LT, pre-op ACD and achieved effective lens position. ACD: Anterior chamber depth, LT: Lens thickness.
Predicted ELP among subjects with ALCON ACRYSOF IQ (SN60WF) lens was 5.22 ± 0.31 and achieved ELP was 4.71 ± 0.48 and significant difference was present (P < 0.001). The mean pre-operative ACD was 2.95 ± 0.36 mm and postoperative ACD was 4.71 ± 0.48 mm. In subjects with Tecnis1 (ZCB00) lens, predicted ELP was 5.33 ± 0.25 and achieved ELP was 5.07 ± 0.44 and significant difference was present (P < 0.001). The mean pre-op ACD was 3.18 ± 0.33 mm and post op ACD was 5.07 ± 0.44 mm. In subjects with ZEISS CT LUCIA (611P) lens, predicted ELP was 5.28 ± 0.25 and achieved ELP was 5.16 ± 0.36 and no significant difference in predicted ELP and achieved ELP (P = 0.069) was noted. The mean pre-op ACD was 3.070 ± 0.317 mm and post-op ACD was 5.164 ± 0.364 mm [Table 2 and Figure 2].
| Parameter | Acrys of IQ (SN60WF) | Tecnis 1 (ZCB00) | CT Lucia (611P) |
|---|---|---|---|
| Predicted ELP | 5.221 | 5.336 | 5.280 |
| Achieved ELP | 4.714 | 5.078 | 5.164 |
ELP: Effective lens position, IOLs: Intra-ocular lens
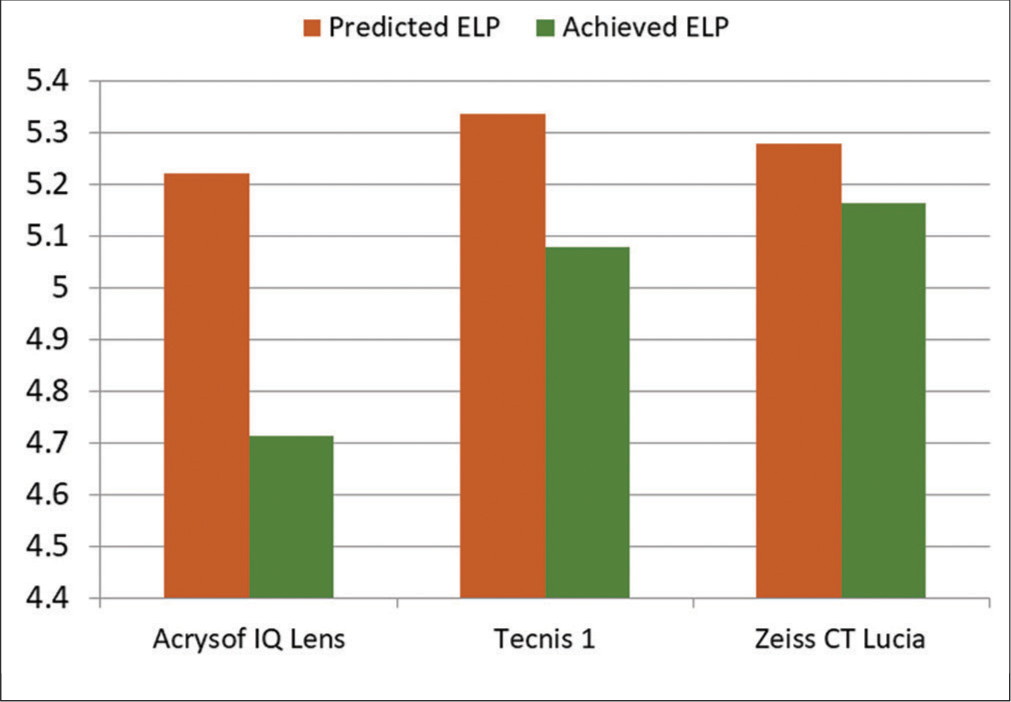
- Difference in predicted ELP and achieved ELP of different IOLs. ELP: Effective lens position, IOLs: Intra-ocular lens.
In the study, predicted residual refraction was −0.12 ± 0.19 and achieved residual refraction (spherical equivalent = Spherical error + ½ cylindrical error) was 0.001 ± 0.35 and there was a statistically significant difference between them. In subjects with ALCON ACRYSOF IQ (SN60WF) lens, predicted residual refraction was −0.14 ± 0.19 and achieved residual refraction was −0.10 ± 0.30 and no significant difference was noted (P = 0.316). In subjects with Tecnis1 (ZCB00) lens, predicted residual refraction was−0.109 ± 0.18 and achieved residual refraction was −0.012 ± 0.28 and there was no significant difference (P = 0.072), but in subjects with Zeiss CT Lucia, (611P) predicted residual refraction was −0.103 ± 0.19 and achieved residual refraction was 0.396 ± 0.22 and significant difference was noted (P < 0.001) [Table 3 and Figure 3].
| Parameter | Acrys of IQ (SN60WF) | Tecnis 1 (ZCB00) | CT Lucia (611P) |
|---|---|---|---|
| Predicted residual refraction | −0.149 | −0.109 | −0.103 |
| Achieved residual refraction | −0.104 | −0.012 | 0.396 |
Comparison of ACD+1/2 LT, pre-op anterior chamber depth and achieved effective lens position. IOLs: Intraocular lens
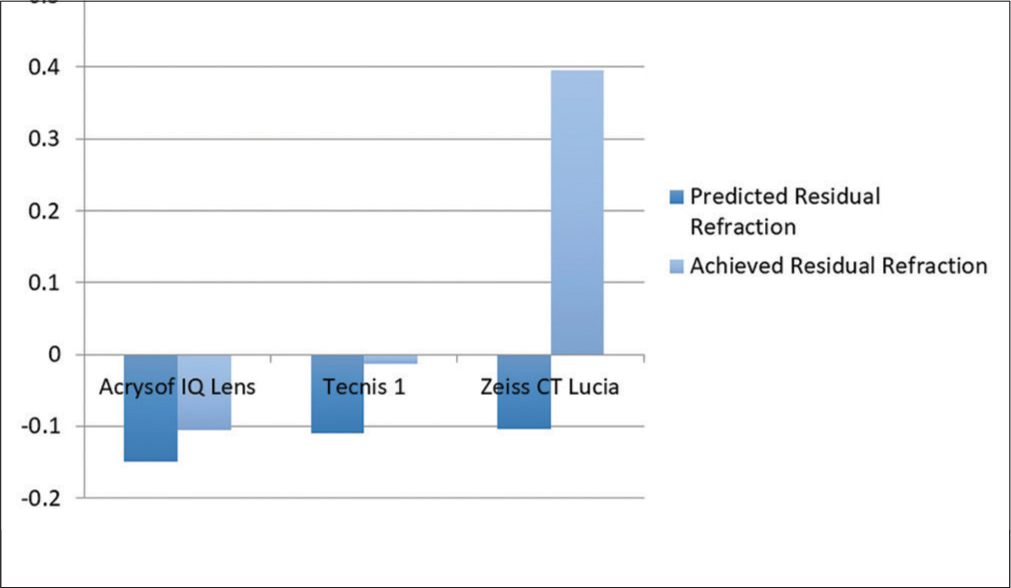
- Difference in predicted residual refraction and achieved residual refraction of different intra-ocular lens.
DISCUSSION
The importance of avoiding refractive surprises lies in patients’ high expectations for their outcomes after cataract surgery. Even though all the biometry measurements are carried out, refractive corrections postoperatively are often needed and the residual refraction of 9–20% of patients is more than 1 D.[15] It is believed that among the factors affecting postoperative visual function, the stability of the post-operative IOL position has been recently considered to be one of the key factors that can be represented by ELP[16] and this means, improvement in refractive outcome requires better methods for predicting ELP. The distance from the anterior apex of the cornea to the mid-sagittal plane of the IOL optic defined as ELP may provide a more accurate model. The final position (the principal plane) of the on-angulated biconvex IOL within the capsular bag should closely approximate the mid-sagittal plane of the phakic lens. Lens grows throughout life leading to decrease in ACD and increase in LT, whereas the distance from the corneal apex to the mid-sagittal plane of the cataractous lens should be relatively unchanged. Therefore, for accurate prediction of ELP the hypothesis that half the thickness of the cataractous lens in addition to ACD will be helpful.[17,18] Measurements of ACD to the anterior surface of the lens are affected by LT, which the proposed parameter aims to overcome. This study signifies that ELP prediction is best achieved by considering ACD + ½ LT rather than just ACD into account and improves estimations of the post-operative position of the IOL principal plane as already proved by previous studies by Chui and Ong.[17]
The other decisive thing, which plays an important role, is the IOL formula. Barrett II universal formula uses all the parameters such as LT, ACD, and WTW and our study further reconfirm that using this formula is best to calculate IOL power which precisely estimates ELP. This study strengthens the hypothesis that ELP prediction based on the position of the mid-sagittal plane of the natural lens reduces inaccuracies when compared to ACD alone and it also allows for individualisation of ELP predictions by taking into account variations in LT.
We calculated two prediction formulae for ELP, first formula was ACD+ ½ LT and the second was ACD alone and mean prediction error that is difference between the mean of predicted ELP and achieved ELP was calculated. In our study, we found that there was statistically significant difference in predicting ELP with ACD + ½ LT and with ACD alone and difference of mean of ACD+½ LT (5.28) and mean of ACD alone (3.07) when compared to achieved ELP (4.99) was 0.29 and 1.92 in two predicting groups respectively. Thus we found that best predictor in ELP is given by ACD + ½ LT which is closer to achieved ELP as compared to ACD and significant reduction in residual prediction error can be achieved by addition of half thickness of lens to ACD. The prediction error by using ACD + ½ LT is reduced but not eliminated, which can further be reduced by correction factor and optimisation of IOL constants used in IOL power calculation formulae. Thus, ELP prediction with ACD + ½ LT (5.28) was estimated higher than achieved ELP (4.99), whereas ACD alone (3.07) was estimated lower than achieved ELP.
The final position of IOL inside the eye depends on various other factors like IOL material, thickness, and optic-haptic configuration. Iwase et al. reported that the eyes which received silicone IOLs had statistically significant myopic shift on follow-up period with a mean shift of −0.53 D and shortened ACD as compared to PMMA, and acrylic IOLs.[19] Koeppl et al.[20] reported that the angulated 3-piece acrylic IOLs showed significant forward movement over the first post-operative 6 months, but the change in refraction was small. Furthermore, optic edge design has an impact on postoperative axial optic movement and ELP, with the sharp edge causing significantly more pronounced backward movement between 1 week and 1 year. The amount and scatter of postoperative movement were greater with the angulated IOL than with the non-angulated models.[21] However, the three IOL included in our study has same IOL design, same material with same optic and haptic dimensions with overall diameter of 6 and 13 mm, respectively, and 0-degree haptic angulation except for haptic design of ZEISS CT LUCIA (611P) which has step vault C type design. On further comparison among the three IOL, we found that ALCON ACRYSOF IQ (SN60WF) and TECNIS1 showed significant differences between predicted and achieved ELP whereas ZEISS CT LUCIA (611P) did not show significant difference. This study shows that all the three IOL lies more anteriorly than predicted position. Among them ZEISS CT LUCIA (611P) is closer to the achieved ELP with difference of only 0.114 which is proven statistically not significant (P = 0.069).
Studies have shown that in short eyes there was largest change in iris position and in longer eyes there was largest change in the lens versus the IOL position and it was noted that IOL moved back from Iris[22] but our study included eyes with AL in normal range.
Residual refraction predicted by IOL master 700 and achieved residual refraction at end of 1 month post-operative showed no significant difference in ALCON ACRYSOF IQ (SN60WF) and TECNIS1 and had myopic shift of −0.10 and −0.11, respectively, but in ZEISS CT LUCIA (611P) predicted was −0.103 ± 0.20 whereas achieved was + 0.396 ± 0.22 showing a total hyperopic shift of 0.5D. Our study concludes that ELP is best predicted by formulae ACD + ½ LT than ACD alone with mean prediction error difference of 0.29 and 1.92 in two groups and it also shows that ALCON ACRYSOF IQ (SN60WF) and J and J TECNIS 1 (ZCB00) are located more anteriorly whereas ZEISS CT LUCIA (611P) is located relatively posteriorly among the three IOLs, these values were clinically and statistically significant. Causative factor that might be leading to hyperopic shift is thickness of IOL, Zeiss IOL is thicker (0.9–1 mm) as compared to J and J TECNIS 1 (ZCB00) (0.7 mm) and ACRYSOF (0.6 mm) leading to hyperopic refractive error and another contributing factor, possibly the preloaded IOL of ZEISS CT LUCIA (611P) flexes midway at the haptics rather than at the optic haptic junction as compared to the other two IOLs. Compared to other studies for prediction of ELP using different hypotheses and algorithms, ours is simpler and customised A constant can help achieve refractive outcomes better.
CONCLUSION
This study concludes that ELP is best predicted by taking into consideration ACD + ½ LT than ACD alone with mean prediction error of 0.29 and 1.92 in the two prediction groups respectively. ALCON ACRYSOF IQ (SN60WF) and TECNIS 1(ZCB00) IOL had myopic shifts of 0.10 and 0.11, respectively, while ZEISS CT LUCIA (611P) had hyperopic shift of 0.5D, as ALCON ACRYSOF IQ (SN60WF) and TECNIS 1 lie more anteriorly and ZEISS CT LUCIA (611P) lies relatively posteriorly among the three IOLs. The residual refraction was significant both clinically and statistically in Zeiss IOL. The reason may be because ALCON ACRYSOF IQ (SN60WF) and TECNIS 1 (ZCB00) IOL have 0-degree haptic angulation for haptic design, but ZEISS CT LUCIA (611P) has step vault C type haptic design. Thus, inclusion of customised A constant in newer formulae like Barrett’s universal II in IOL power calculation reduces prediction error and helps to reduce post-operative refractive surprise. Nevertheless, this study has certain limitations like sample size were small, only uncomplicated cataract cases were included, and extremes of AL s were not taken (AL between 22.00 mm and 24.50 mm were included). Similar study conducted with larger sample size and inclusion of complicated cataract and extreme AL in future can give better prediction of ELP and shall help to refine normogram of ELP in calculation of IOL power.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Conflicts of interest
Dr Sri Ganesh is a consultant to Carl Zeiss Meditec.
Financial support and sponsorship
Nil.
References
- The Hoffer Q formula: A comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19:700-12.
- [CrossRef] [Google Scholar]
- Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 1992;18:125-9.
- [CrossRef] [Google Scholar]
- Calculation of intraocular lens power: A review. Acta Ophthalmol Scand. 2007;85:472-85.
- [CrossRef] [Google Scholar]
- Accuracy of intraocular lens power calculation using anterior chamber depth from two devices with Barrett universal II formula. J Ophthalmol. 2019;2019:8172615.
- [CrossRef] [Google Scholar]
- Barrett Universal II Formula Singapore: Asia-Pacific association of Cataract and Refractive Surgeons; 2018.
- [Google Scholar]
- An improved universal theoretical formula for intraocular lens power prediction. J Cataract Refract Surg. 1993;19:713-20.
- [CrossRef] [Google Scholar]
- Measurement and use of postoperative anterior chamber depth of fellow eye in refractive outcomes. J Cataract Refract Surg. 2015;41:778-84.
- [CrossRef] [Google Scholar]
- Effect of centration and circularity of manual capsulorrhexis on cataract surgery refractive outcomes. Ophthalmology. 2014;121:763-70.
- [CrossRef] [Google Scholar]
- Relationship between effective lens position and axial position of a thick intraocular lens. PLoS One. 2018;13:e0198824.
- [CrossRef] [Google Scholar]
- A three-part system for refining intraocular lens power calculations. J Cataract Refract Surg. 1988;14:17-24.
- [CrossRef] [Google Scholar]
- Intraocular lens power calculation with an improved anterior chamber depth prediction algorithm. J Cataract Refract Surg. 1995;21:313-9.
- [CrossRef] [Google Scholar]
- IOL calculation using paraxial matrix optics. Ophthalmic Physiol Opt. 2009;29:458-63.
- [CrossRef] [Google Scholar]
- Estimation of effective lens position using a method independent of preoperative keratometry readings. J Cataract Refract Surg. 2011;37:506-12.
- [CrossRef] [Google Scholar]
- Prediction of effective lens position using multiobjective evolutionary algorithm. Transl Vis Sci Technol. 2019;8:64.
- [CrossRef] [Google Scholar]
- Predicting the postoperative intraocular lens position using continuous intraoperative optical coherence tomography measurements. Invest Ophthalmol Vis Sci. 2013;54:5196-203.
- [CrossRef] [Google Scholar]
- Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008;34:368-76.
- [CrossRef] [Google Scholar]
- Improving the prediction of effective lens position for intraocular lens power calculations. Asian J Ophthalmol. 2020;17:233-42.
- [CrossRef] [Google Scholar]
- Anterior chamber depth-a predictor of refractive outcomes after age-related cataract surgery. BMC Ophthalmol. 2019;19:134.
- [CrossRef] [Google Scholar]
- Postoperative refraction changes in phacoemulsification cataract surgery with implantation of different types of intraocular lens. Eur J Ophthalmol. 2008;18:371-6.
- [CrossRef] [Google Scholar]
- Postoperative change in effective lens position of a 3-piece acrylic intraocular lens. J Cataract Refract Surg. 2003;29:1974-9.
- [CrossRef] [Google Scholar]
- Effect of optic edge design and haptic angulation on postoperative intraocular lens position change. J Cataract Refract Surg. 2004;30:52-7.
- [CrossRef] [Google Scholar]
- Anterior chamber depth and iris and lens position before and after phacoemulsification in eyes with a short or long axial length. J Cataract Refract Surg. 2016;42:563-8.
- [CrossRef] [Google Scholar]







