Translate this page into:
Aspheric hydrophobic acrylic intraocular lens outcomes in patients with additional ocular pathology
*Corresponding author: James Redmayne, School of Medicine, Griffith University, Southport, Australia. j.redmayne@griffith.edu.au
-
Received: ,
Accepted: ,
How to cite this article: Redmayne J, Preston S, Moore S. Aspheric hydrophobic acrylic intraocular lens outcomes in patients with additional ocular pathology. Glob J Cataract Surg Res Ophthalmol. 2024;3:16-20. doi: 10.25259/GJCSRO_31_2023
Abstract
Objectives:
The objective of the study was to examine the real-world visual performance, refractive outcomes, and glistening occurrence of a hydrophobic acrylic aspheric monofocal intraocular lens (IOL) in patients with ocular comorbidities.
Materials and Methods:
All cases of cataract surgery with the implantation of a hydrophobic acrylic aspheric monofocal IOL in a single centre between September 2020 and March 2022 were reviewed in a retrospective cohort study. Refraction (autorefract), visual acuity, peri- and post-operative complications, and the presence of posterior capsular opacification (PCO) and glistenings were recorded. Due to facility and surgeon preference, this lens was utilised predominantly for younger patients with additional ocular pathology.
Results:
Data regarding 15 patients (21 eyes) were collected. The mean age was 57.3 years (Range: 37–70). Eleven patients (73%) had ocular pathology in addition to cataracts. One patient was excluded due to a lack of follow-up. Post-operative refraction was available for 13 eyes (61%). The median follow-up time was five months. Corrected distance visual acuity (logarithm of the minimum angle of resolution) improved from 0.52 ± 0.54 to 0.17 ± 0.21 (mean ± standard deviation; P = 0.01); 95% confidence interval 0.08–0.26). 77% of eyes were within 0.5 D of the refractive target, and 92% were within 0.7 D. Six patients (30%) had documented PCO, with two requiring YAG capsulotomy. No patients had IOL glistenings.
Conclusion:
The hydrophobic aspheric monofocal IOL performs well for young patients with ocular pathology in addition to cataracts. Further, long-term follow-up will help to support its use for patients who require greater than standard longevity from an IOL.
Keywords
Intraocular lens
Cataract
Capsule opacification
Macular oedema
Complications
INTRODUCTION
Cataract surgery is the most common surgical procedure performed in developed countries and usually requires an artificial intraocular lens (IOL) to be implanted.[1] With an ever-increasing selection of artificial lenses available, an evidence-based approach to selecting an optimal IOL for each patient requires careful consideration.
The average age of patients undergoing cataract surgery is ~70 years.[2,3] However, IOL implantation is becoming more frequent in younger patients. Due to the greater life expectancy in younger patients, IOLs used in this population will need to maintain their optical clarity for a longer duration. While cataract risk is associated with increasing age, other ocular pathologies and their treatments can contribute to the early onset of cataracts.[4,5] Prior vitrectomy and steroid exposure, as seen in uveitis or diabetic maculopathy patients, have been implicated in early cataract development.[6,7] As such, a large portion of younger patients with visually significant cataracts have additional ocular pathology.[8]
Although infrequent, complications affecting vision can occur following cataract surgery.
Posterior capsular opacification (PCO) is the most common complication of cataract surgery, with younger patients at significantly higher risk.[9,10] PCO can cause significant visual symptoms and can be effectively treated with laser capsulotomy. IOL morphology has been shown to affect the development of PCO and modern lens designs are engineered to reduce rates of PCO.[11] Glistenings are water-filled vacuoles that form within IOLs as a result of material degradation, most commonly seen in hydrophobic acrylic lenses.[12] High densities of glistenings are thought to cause light scatter and have been shown to increase over time; however, the impact on visual outcomes is unclear.[13]
The Clareon monofocal IOL (Alcon Vision LLC, Fort Worth, TX, USA) is a hydrophobic, square-edged, and acrylate/methacrylate copolymer lens that has been demonstrated to have low rates of glistenings and PCO.[14-18] In our setting, a public hospital ophthalmology department in regional New Zealand, the Clareon monofocal IOL has been utilised since September 2020 for younger cataract patients. Previously published studies analysing the performance of the Clareon IOL exclude patients with additional ocular pathology that could affect visual outcomes – presumably with the goal of reducing confounding variables.[14-18] We completed a retrospective cohort study to examine the real-world performance of the Clareon monofocal IOL. To the best of our knowledge, this is the first study to include and focus on patients with additional ocular pathology in a patient group significantly younger than the average cataract patient.
MATERIALS AND METHODS
A retrospective review was performed to assess the visual outcomes for all patients undergoing cataract surgery with Clareon IOL implantation by a single surgeon between September 2020 and March 2022. Patients receiving alternative IOL implants were excluded from the study. Importantly, patients with additional ocular pathology were not excluded from the study. A Human Research Ethics Committee granted ethics approval, and the study adhered to the tenets of the Declaration of Helsinki. A single surgeon performed cataract surgery. The clinical records of all included patients were reviewed. Data collected were age, sex, date of surgery, length of follow-up, IOL power, pre-and post-operative visual acuity (logarithm of the minimum angle of resolution [logMAR]), pre-and post-operative refraction (dioptres), ocular comorbidities, intra-and post-operative complications, including PCO, cystoid macular oedema (CMO), glistenings and requirement for ND: YAG Capsulotomy. Refraction was obtained through Autorefract (Tonoref II, Nidek, Japan). CMO was assessed through macula optical coherence tomography (OCT) (DRI OCT Triton Plus, TOPCON, Tokyo, Japan). Slit-lamp biomicroscopy was performed at each clinical review.
Data were entered into a Microsoft Excel Spread sheet (Microsoft Corp, Washington, USA). Visual acuity was converted from Snellen chart findings to logMAR units for statistical analysis using the techniques described by Moussa et al.[19] Statistical analysis was performed through Microsoft Excel, with standardised graphs for reporting refractive outcomes prepared as detailed by Dupps et al.[20] Continuous variables were expressed as mean values ± standard deviations and categorical variables were expressed as individual counts. Paired t-tests were used for comparative measures, with P < 0.05 considered statistically significant.
RESULTS
Fifteen patients (20 eyes) were identified to have undergone cataract surgery with the Clareon IOL within the study period. One patient was excluded due to lack of follow-up data. The mean age was 57.1 years (range 37–70). Seven patients were female (46%). Full baseline patient characteristics are summarised in Table 1. Sixteen eyes (80%) had ocular pathology in addition to cataracts [Table 2]. No intraoperative complications were recorded.
| Characteristic | Value | n |
|---|---|---|
| Sex (M: F) | 8:7 | 20 |
| Side (right/left) | 11/9 | 20 |
| Mean age (range) | 57.1 (37, 70) | 20 |
| Mean pre-operative CDVA (logMAR) | 0.52 (±0.53) | 20 |
| Mean SE refractive error (D) | −3.3 (±3.6) | 12 |
| Mean target refraction (D) | −0.18 (±1.3) | 20 |
LogMAR: Logarithm of the minimum angle of resolution, CDVA: Corrected distance visual acuity, SE: Spherical equivalent
| Pathology | Number of patients (%) |
|---|---|
| Fuchs | 1 (5) |
| Corneal scarring | 1 (5) |
| BRAVO | 1 (5) |
| Chorioretinal atrophy | 1 (5) |
| T2DM w/o DR | 1 (5) |
| DR/DMO | 2 (10) |
| High myopia | 2 (10) |
| POAG | 2 (10) |
| Uveitis | 2 (10) |
| Keratoconus | 3 (15) |
| PKP | 3 (15) |
| Previous RD | 3 (15) |
| Nil | 4 (20) |
BRVO: Branch retinal vein occlusion, T2DM, Type 2 diabetes mellitus, POAG: Primary open-angle glaucoma, PKP: Penetrating keratoplasty, DMO: Diabetic macular oedema , DR: Diabetic retinopathy, RD: Retinal detachment
Post-operative outcomes are detailed in Table 3. Across the whole group, post-operative corrected distance visual acuity (CDVA) was 20/40 or better for 18 eyes (90%), 20/30 or better for 16 eyes (80%) and 20/20 or better for 4 eyes (20%). Mean pre-operative CDVA was 20/60 (0.52 logMAR [±0.53]), which improved to 20/30 (0.17 logMAR [±0.21]) post-operatively (P = 0.01, 95% confidence interval [CI]; 0.07 to 0.26). Comparative unaided distance visual acuity (UDVA) data were available for seven eyes. Mean pre-operative UDVA was 20/160 (0.99 loMAR [±0.53]), which improved postoperatively to 20/40 (0.31 logMAR [±0.21]), (P = 0.03, 95% confidence interval [CI]; 0.03–0.59).
| Characteristic | Value (SD) | n |
|---|---|---|
| Mean post-operative CDVA (logMAR) | 0.17 (±0.21) | 20 |
| Mean SE refractive error (D) | −0.91 (±3.03) | 13 |
| Mean residual cylinder (D) | −1.17 (±1.47) | 13 |
| SE within 0.5D (%) | 10 (77%) | 13 |
LogMAR: Logarithm of the minimum angle of resolution, CDVA: Corrected distance visual acuity, SD: Standard deviation, SE: Spherical equivalent
Paired pre- and post-operative refractive data were available for 13 eyes, allowing analysis as per the standard graphs for reporting outcomes of refractive surgery [Figure 1].[20] Refractive error (Spherical equivalent) was within ±0.5 D in 10 eyes (77%) and within ±1.0 D in 10 eyes (92%). Mean refractive error improved from −3.31 D (±3.56, 95% CI; −5.33–−1.3) to −0.91D (±3.03, 95% CI; −2.56–0.73).
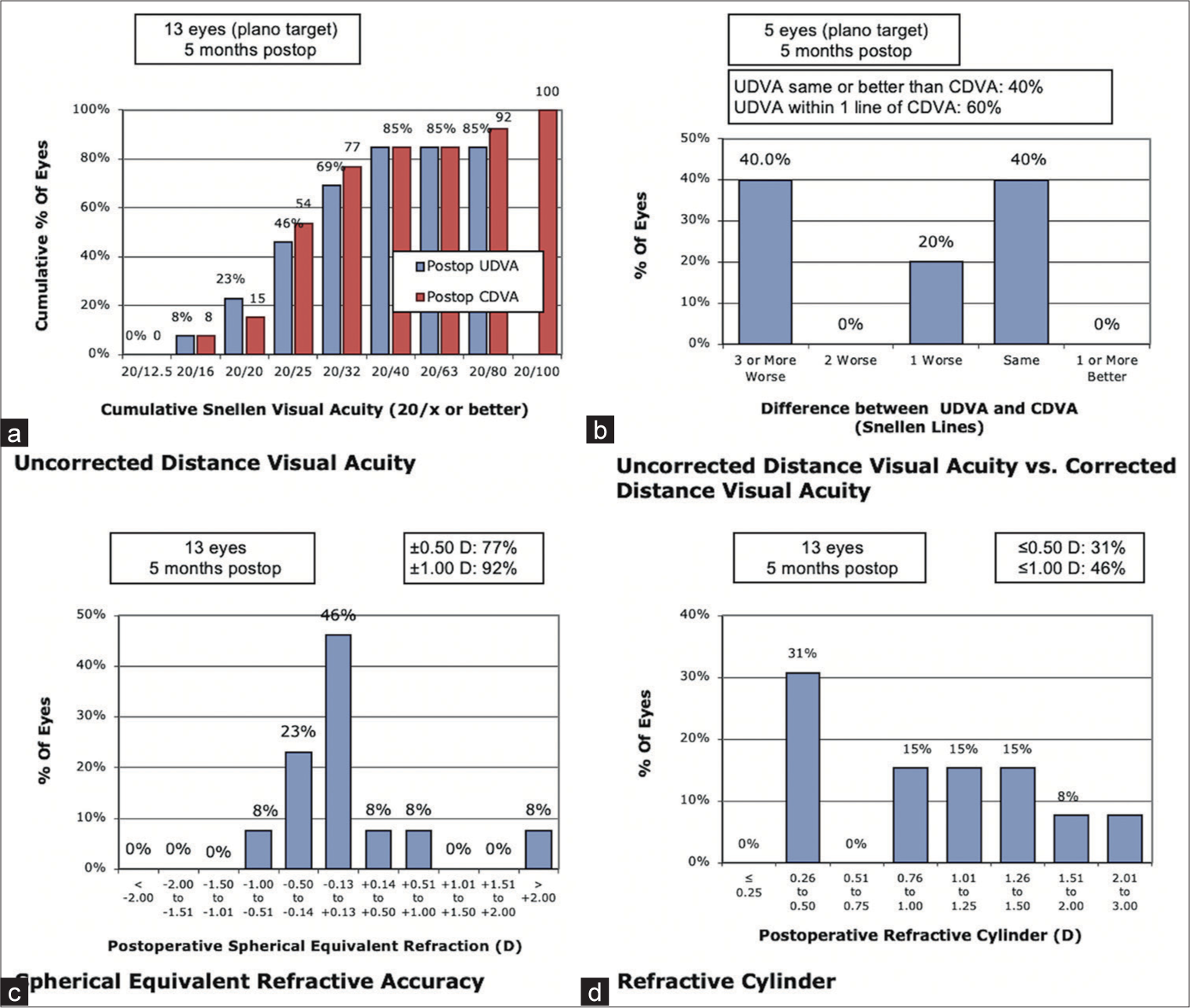
- Refractive outcomes demonstrating (a) cumulative post-operative unaided and corrected distance visual acuity, (b) Difference between unaided and corrected distance visual acuity, (c) postoperative refraction (spherical equivalent), and (d) post-operative cylindrical error. UDVA: Uncorrected distance visual acuity, CDVA: Corrected distance visual acuity
Adverse events are detailed in Table 4. Post-operative CMO was recorded in 3 eyes (15%), two of which had a history of retinal detachment and one with a history of branch retinal vein occlusion. PCO was documented in 6 eyes (30%); however, laser capsulotomy was only required for two eyes (10%). CMO had been documented in both eyes that required laser capsulotomy, suggesting an inflammatory contribution to PCO formation. Lens glistenings were not seen in any patients.
| Event | Events (%) |
|---|---|
| Intraoperative complications | 0 (0) |
| CMO | 3 (15) |
| PCO | 6 (30) |
| YAG PC | 2 (10) |
| Glistenings | 0 (0) |
PCO: Posterior capsular opacification, CMO: Cystoid macular oedema, YAG PC: Yttrium-aluminium-garnet laser posterior capsulotomy
DISCUSSION
This study details the real-world outcomes of the Clareon IOLs in a young cohort of patients, predominantly with additional ocular pathology. The Clareon lens has been reported to have reduced rates of glistenings, a desirable trait for use in younger patients where increased life expectancy places greater importance on maintaining optical clarity for an extended period.[14,21]
A number of studies have demonstrated the Clareon hydrophobic acrylic aspheric monofocal IOL to provide good visual and refractive outcomes, with low rates of glistenings and posterior capsule opacification in eyes without additional ocular pathology.[14-18] In a study of 110 eyes, Oshika et al. demonstrated CDVA of 20/30 or better in all 110 (100%) eyes and 20/20 or better in 101–105 (91.8–95.5%) eyes, at 1 week and 12 months postoperatively.[14] Refractive error was within ±1.0 D in 100 (90.9%) of 110 eyes. No glistenings were seen at 12 months.[14] Stanojcic et al. demonstrated post-operative CDVA of 20/30 or better in 99% of eyes and 20/20 or better in 81% of eyes (n = 68).[18] Refractive error was within ±1.0 D in 97% of eyes and within ±0.5 D in 80% of eyes.[18] PCO was seen in 7% of eyes, but Nd: YAG capsulotomy was only required in 2%. CMO was seen in 4.3% of eyes. Glistenings were seen in 5% of lenses; however, the authors propose that scratches induced by a specific IOL delivery system may have contributed to these glistenings.[18] Lehmann et al. studied 350 eyes and demonstrated CDVA of 20/40 or better in 99.7% of eyes and 20/20 or better in 86.8%. Refractive error was within ±1.0 D in 99% of eyes and within ±0.5 D in 85% of eyes at 12-month follow-up. PCO was seen in 5.4% of eyes and CMO in 1.1%. No glistenings were seen at 12 months.[17]
Our study is the first, to the best of our knowledge, that investigates the performance of this lens in patients with additional ocular pathology. Similar to the above studies, refractive error was within ±1.0 D in 91% of eyes. Postoperative visual acuity outcomes were predictably reduced compared to the previous literature, with a large portion (90%) of patients achieving CDVA >20/40 but few patients (20%) achieving CDVA of 20/20 or greater. Given the good refractive predictability, we believe that this reflects the impact of coexisting ocular pathology on visual potential rather than the reduced performance of the lens in comorbid eyes.
Our findings demonstrate greater CMO rates (15%) than previous studies with this lens (1.1–4.3%); however similar rates when compared to previous studies in eyes with additional ocular pathology.[17,18] The incidence of Pseudophakic CMO after modern cataract surgery is estimated to be between 0.1% and 3.8%; however is increased to as high as 5–28% in those with retinal vein occlusion, non-infectious-uveitis or prior vitrectomy.[22] As all patients in our cohort who experienced CMO had a history of previous venous occlusion or vitrectomy, we believe our CMO rates relate to underlying pathology rather than lens factors.
The rates of PCO (30%) in our cohort were greater than documented rates in previous studies (5.4–7%).[17,18] Drawing conclusions from our PCO rates is challenging, as there is no gold standard method for quantifying capsular opacification. [23] PCO in our study was identified through dilated slit-lamp examination and was documented as either present or not present, without comment on visual significance. Two previous studies that documented PCO rates with the Clareon lens utilised different systems of grading PCO location and visual significance, which may account for the difference in rates.[17,18] As PCO formation is likely linked to post-operative inflammation, our increased PCO rates may relate to increased inflammation mediated by ocular comorbidities.[24] Unfortunately, meaningful interpretation of PCO rates from our data is limited due to our small patient numbers.[18]
This study is limited by its retrospective design and small sample size. Unfortunately, sample size is a common limitation in real-world studies investigating cataract surgery outcomes in specific patient populations. We believe our findings add to the current literature despite the small sample, given prior studies have excluded patients with additional ocular pathology. Future prospective studies with greater sample size and objective measures for quantification of PCO and glistenings would provide further insight into lens performance in this population.
CONCLUSION
When used in young cataract patients with additional ocular pathology, the Clareon IOLs implant has high refractive predictability and provides good visual outcomes, allowing for pre-existing limitations in visual potential. Further studies with greater patient numbers are required to establish better PCO and CMO rates associated with this lens within this population group.
Ethical approval
Ethical approval for this study was granted by the Griffith University Human Research Ethics Committee (GUHREC), Queensland, Australia. GU Ref No: 2022/850. Date of approval: 9 Dec 2022.
Declaration of patient consent
Patient’s consent is not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Health care utilisation: Surgical procedures. Paris: OECD; Available from: https://stats.oecd.org/index.aspx?queryid=30167 [Last accessed on 2023 May 24]
- [Google Scholar]
- Demographics and ocular biometric characteristics of patients undergoing cataract surgery in Auckland, New Zealand. Clin Exp Ophthalmol. 2016;44:106-13.
- [CrossRef] [PubMed] [Google Scholar]
- Geographic variation in the rate and timing of cataract surgery among US communities. JAMA Ophthalmol. 2016;134:267-76.
- [CrossRef] [PubMed] [Google Scholar]
- External factors in the development of cataract. Eye (Lond). 2005;19:1074-82.
- [CrossRef] [PubMed] [Google Scholar]
- Development of cataract and associated risk factors: The visual impairment project. Arch Ophthalmol. 2006;124:79-85.
- [CrossRef] [PubMed] [Google Scholar]
- Surgery for post-vitrectomy cataract. Cochrane Database Syst Rev. 2013;12:CD006366.
- [CrossRef] [PubMed] [Google Scholar]
- A study to explore the risk factors for the early onset of cataract in India. Eye (Lond). 2010;24:686-94.
- [CrossRef] [PubMed] [Google Scholar]
- Retrospective analyses of potential risk factors for posterior capsule opacification after cataract surgery. J Ophthalmol. 2018;2018:9089285.
- [CrossRef] [PubMed] [Google Scholar]
- 5 year incidence of YAG capsulotomy and PCO after cataract surgery with single-piece monofocal intraocular lenses: A real-world evidence study of 20,763 eyes. Eye (Lond). 2020;34:960-8.
- [CrossRef] [PubMed] [Google Scholar]
- Intraocular lens optic edge design for the prevention of posterior capsule opacification after cataract surgery. Cochrane Database Syst Rev. 2021;8:CD012516.
- [CrossRef] [PubMed] [Google Scholar]
- Subjective visual performance and objective optical quality with intraocular lens glistening and surface light scattering. J Refract Surg. 2018;34:372-8.
- [CrossRef] [PubMed] [Google Scholar]
- A review of late intraocular lens opacifications. Curr Opin Ophthalmol. 2021;32:31-44.
- [CrossRef] [PubMed] [Google Scholar]
- Mid-term and long-term clinical assessments of a new 1-piece hydrophobic acrylic IOL with hydroxyethyl methacrylate. J Cataract Refract Surg. 2020;46:682-7.
- [CrossRef] [PubMed] [Google Scholar]
- Twelve-months follow-up postmarket study of a hydrophobic intraocular lens using a preloaded automated injector in an Indian population. Clin Ophthalmol. 2022;16:4215-25.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of visual outcomes and patient satisfaction following cataract surgery with two monofocal intraocular lenses: Clareon® AcrySof® IQ monofocal. Open Ophthalmol J. 2021;15:144.
- [CrossRef] [Google Scholar]
- Effectiveness and safety of the clareon monofocal intraocular lens: Outcomes from a 12-month single-arm clinical study in a large sample. Clin Ophthalmol. 2021;15:1647-57.
- [CrossRef] [PubMed] [Google Scholar]
- Visual and refractive outcomes and glistenings occurrence after implantation of 2 hydrophobic acrylic aspheric monofocal IOLs. J Cataract Refract Surg. 2020;46:986-94.
- [CrossRef] [PubMed] [Google Scholar]
- A novel excel sheet conversion tool from Snellen fraction to LogMAR including 'counting fingers', 'hand movement', 'light perception' and 'no light perception' and focused review of literature of low visual acuity reference values. Acta Ophthalmol. 2021;99:e963-5.
- [CrossRef] [PubMed] [Google Scholar]
- Standardized graphs and terms for refractive surgery results. J Cataract Refract Surg. 2011;37:1-3.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. J Cataract Refract Surg. 2019;45:1490-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cystoid macular oedema following cataract surgery: A review. Clin Exp Ophthalmol. 2019;47:346-56.
- [CrossRef] [PubMed] [Google Scholar]
- Systems of analysis of posterior capsule opacification. Br J Ophthalmol. 2002;86:1181-6.
- [CrossRef] [PubMed] [Google Scholar]
- Role of cytokines in the pathogenesis of posterior capsule opacification. Br J Ophthalmol. 2000;84:332-6.
- [CrossRef] [PubMed] [Google Scholar]







